The effect of ischemic preconditioning on nitric oxide synthase activity during myocardial ischemia and reperfusion in anesthetized dogs
- *Corresponding Author:
- Prof Dr Ágnes Végh
Department of Pharmacology and Pharmacotherapy, University of Szeged, Albert Szent-Györgyi Faculty of Medicine, Dóm tér 12, PO Box 427, H-6720 Hungary.
Tel:36-62-545-673
Fax:36-63-545-680
E-mail:vegh.agnes@med.u-szeged.hu
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact support@pulsus.com
[ft_below_content] =>Keywords
Ischemia/reperfusion; Nitrate/nitrite; NOS activity; Preconditioning; Superoxide
The first evidence that nitric oxide (NO) plays an essential role in the cardioprotective effects of preconditioning (PC) resulted from studies involving anesthetized dogs, which demonstrated that the marked antiarrhythmic effect of PC, induced by brief periods of coronary artery occlusion, were abolished when L-NG-nitroarginine methyl ester, an inhibitor of NO formation, was administered either before the PC procedure or immediately before a prolonged (25 min) ischemic insult [1]. These results also suggested that NO may play both a trigger and mediator role in this antiarrhythmic protection. More recently, we have provided further evidence of this by measuring the plasma concentrations of NO metabolites (nitrate and nitrite, NOx) in the blood of the coronary sinus [2]. The results showed that PC elevated NOx levels, and these were maintained or even further increased during prolonged occlusion, in contrast to the nonpreconditioned controls, in which NOx, after an initial increase, was substantially reduced [2].
There remains ongoing debate regarding whether the generation of NO increases or decreases during myocardial ischemia, and whether the protection provided by PC is due to a reduction or an elevation of NO bioavailability. A possible explanation for these controversies may be found in the differences in the experimental methods and in the methods used for NO detection. For example, the results of studies (mainly in vitro studies) using electron paramagnetic resonance spectroscopy for NO detection suggest that the ischemic milieu (low pH, reduced oxygen availability, etc) facilitates the conversion of nitrite to NO, resulting in enhanced formation and accumulation of nonenzymatic NO, which reacts with superoxide to form toxic peroxynitrite [3-5]. Therefore, by attenuating the excess of NO production and subsequent peroxynitrite formation, PC would result in cardioprotection [5]. These findings were, however, not confirmed by the results of the in vivo experiments. Studies involving anesthetized dogs and pigs using methods other than electron paramagnetic resonance spectroscopy (eg, microdialysis and electrochemical techniques) for NO detection indicated that NO production is decreased rather than increased during sustained coronary artery occlusion [6,7]. For example, it has been found that in anesthetized pigs subjected to a 15 min period of occlusion, the release of NO in the interstitial fluid was markedly reduced [7]. Moreover, in isolated rabbit hearts, the release of NO in the coronary sinus was significantly decreased after 60 min of ischemia [8]. Our results in anesthetized dogs have confirmed these findings, but we have also noted that the decrease in NO bioavailability during prolonged ischemia is always preceded by an initial increase in NOx that occurs soon (approximately 7 min) after the onset of the occlusion [2]. We have also reported that the NOx levels are significantly elevated by the brief periods of PC occlusion, and that these levels are maintained or even further increased during subsequent, more prolonged ischemia. We hypothesize that the increased NO bioavailability during ischemia may be important for the antiarrhythmic effect of PC [2].
Because nitric oxide synthase (NOS) activity was not measured in the above-mentioned study, we have designed experiments to examine, using a limited number of dogs, whether changes in NO metabolites during coronary artery occlusion in preconditioned and nonpreconditioned dogs are due to alterations in NOS activity. In parallel, we also determined superoxide and nitrotyrosine (NT) production.
Methods
Ethics statement
The origin and maintenance of the dogs used in the present study were in accordance with Hungarian law (XXVIII, chapter IV, paragraph 31) regarding large experimental animals, which conforms to the Guide for the Care and Use of Laboratory Animals published by the United States National Institutes of Health (NIH Publication No. 85-23, revised 1996), and conformed to the Directive 2010/63/EU of the European Parliament. The protocols were approved by the Ethical Committee for the Protection of Animals in Research of the University of Szeged, Szeged, Hungary (approval number: I-74-5-2012) and by the Department of Animal Health and Food Control of the Ministry of Agriculture and Rural Development (authority approval number XIII/1211/2012).
Surgical preparation
Inbred mongrel dogs of either sex (body weight 16 kg to 20 kg) were used. Before the studies, the dogs were housed in an animal room (temperature 10°C to 20°C; humidity 40% to 70%; lighting 12 h per day; two animals per pen) for at least two weeks and fed a standard diet with ad libitum access to water. Food was withdrawn 24 h before anesthesia. Animals were anesthetized with a mixture of chloralose and urethane (60 mg/kg and 200 mg/kg intravenously, respectively; Sigma, USA), and ventilated with room air using a Harvard respirator at a rate and volume sufficient to maintain arterial blood gases and pH within normal limits [9]. The anesthesia was maintained by the administration of an additional slow injection of the same anesthetics (dose volume of 0.5 mL/kg). Body temperature was recorded at the midesophagus and maintained at 37±0.5°C by a heating pad.
The surgical procedures were similar to those described previously [9]. Briefly, polyethylene catheters were inserted into the right femoral artery for monitoring arterial blood pressure, and into the left ventricle for the measurement of systolic and end-diastolic pressures, and left ventricular dP/dt. The right femoral vein was also catheterized for further administration of anesthetic. Another catheter was positioned in the coronary sinus through the jugular vein, from which blood samples were collected for the measurement of plasma NOx levels.
A thoracotomy was performed at the fifth intercostal space and the left anterior descending artery (LAD) was prepared for occlusion just proximal to the first main diagonal branch. The severity of myocardial ischemia was assessed by changes in epicardial ST segment (mV) and in the degree of inhomogeneity of electrical activation (ms) recorded from the left ventricular wall distal to the occlusion site using a composite electrode, as previously described [9].
Ventricular arrhythmias during a 25 min period of occlusion were analyzed according to the ‘Lambeth Conventions’ [10] with the modification outlined previously [9]. Briefly, the total number of ventricular premature beats (VPBs), the incidence and the number of episodes of ventricular tachycardia (defined as a run of ≥4 VPBs at a rate faster than the resting heart rate), and the incidence of ventricular fibrillation were measured.
All parameters, together with a chest lead electrocardiogram, were monitored using a Plugsys Haemodynamic apparatus (Hugo Sachs Electronics, Germany) and recorded on a Graphtec Thermal Array Recorder (Hugo Sachs Electronics, Germany).
Assessment of plasma nitrate/nitrite levels
Plasma NOx concentrations were determined using the Griess reaction in blood samples collected from the coronary sinus at various time intervals (Figure 1) as described previously [2]. After sample preparation (centrifugation and enzymatic reduction of nitrate to nitrite), the Griess reagent was added to the mixture and incubated for an additional 10 min at room temperature. The absorbance of the azo compound was measured spectrophotometrically at a wavelength of 540 nm and the total NOx concentration (μmol/L) was determined using a standard calibration curve of NaNO2 and NaNO3 (Sigma, USA).
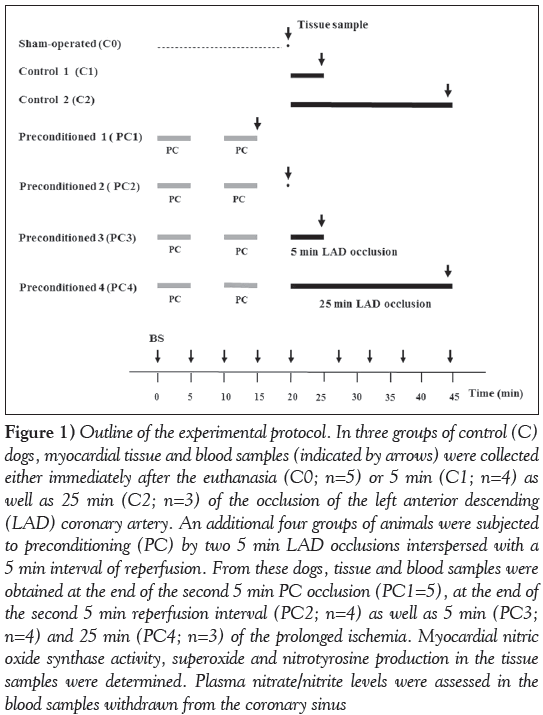
Figure 1: Outline of the experimental protocol. In three groups of control (C) dogs, myocardial tissue and blood samples (indicated by arrows) were collected either immediately after the euthanasia (C0; n=5) or 5 min (C1; n=4) as well as 25 min (C2; n=3) of the occlusion of the left anterior descending (LAD) coronary artery. An additional four groups of animals were subjected to preconditioning (PC) by two 5 min LAD occlusions interspersed with a 5 min interval of reperfusion. From these dogs, tissue and blood samples were obtained at the end of the second 5 min PC occlusion (PC1=5), at the end of the second 5 min reperfusion interval (PC2; n=4) as well as 5 min (PC3; n=4) and 25 min (PC4; n=3) of the prolonged ischemia. Myocardial nitric oxide synthase activity, superoxide and nitrotyrosine production in the tissue samples were determined. Plasma nitrate/nitrite levels were assessed in the blood samples withdrawn from the coronary sinus
Determination of nos activity
NOS activity was measuring using an assay kit (Cayman Chemical, USA) based on the biochemical conversion of [3H] L-arginine to [3H] L-citrulline by NOS, as has been described recently [11]. Briefly, according to the manufacturer’s instructions, total NOS protein was isolated from the myocardial tissue (100 mg), homogenized in ice-cold homogenization buffer (Cayman Chemical, USA) and centrifuged at 2000 g for 15 min. A liquid scintillation counter (Wizard, USA) was used to determine NOS activation by measuring the amount of radiolabelled citrulline. The activity of the enzyme was expressed as the percentage of total counts, corrected using the background counts per minute.
Assessment of myocardial superoxide production
Myocardial tissue blocks (0.5 cm × 0.5 cm × 2.0 cm) were excised from the ischemic LAD region and, after cryosectioning, the samples were stained with dihydroethidium (1 μmol/L, Sigma, USA) as described previously (2,12). Ten to 15 images were obtained from both from the stained samples and the negative controls (incubated with 100 mmol/L N-acetyl-L-cysteine, Sigma, USA) using a confocal microscope (Olympus FV1000, Japan). The intensity of the fluorescent signals was analysed using ImageJ software (National Institutes of Health, USA) and expressed in arbitrary units.
Determination of peroxynitrite production
To assess peroxynitrite production, the formation of NT was measured by Western blot, as previously described [13]. Briefly, after tissue preparation (homogenization and centrifugation), the protein concentration in the supernatant was determined using the method described by Lowry. Following electrophoresis, the proteins were transferred onto polyvinylidene fluoride membrane. After incubation with a monoclonal anti-NT antibody (MAB5404, Chemicon, USA) and horseradish peroxidase-conjugated rabbit anti-mouse immunoglobulin G (P0161, Dakocytomation, Denmark) as the secondary antibody, the membrane was developed using an enhanced chemiluminescence kit (ECL Plus, GE Healthcare, United Kingdom), exposed to x-ray film and scanned. ImageJ was used to determine the density of the NT bands. Equal loading was ensured using Coomassie Blue staining.
Experimental protocol
The experimental protocol is illustrated in Figure 1. The dogs were randomly assigned to one of seven groups, each containing between three and five animals. After surgery, the dogs were allowed to recover for 30 min. In all groups except the sham-operated controls, myocardial ischemia was induced by the occlusion of the LAD. Three groups served as controls. In these groups, after euthanasia by anesthetic overdose, myocardial tissue samples were harvested either at the end of the 30 min recovery period (C0; n=5) or at 5 min (C1; n=4) and 25 min (C2; n=3) of the coronary artery occlusion. In four groups, the dogs were preconditioned using two 5 min occlusions of the LAD, interspersed with a 5 min interval of reperfusion. In these groups, after euthanasia by anesthetic overdose, samples were taken at the end of the second PC occlusion (PC1; n=5), after the PC procedure but just before the 25 min occlusion (PC2; n=4), as well as at 5 min (PC3; n=4) and 25 min (PC4; n=3) of the prolonged ischemia. In each group, tissue samples to be used for the determination of NOS activity and superoxide production were excised from the ischemic area supplied by the occluded LAD. Blood samples were also collected from the coronary sinus at various time points of the experiment for the measurement of NOx levels.
Statistical analysis
Values are expressed as mean ± SEM, and the differences among means were compared using repeated-measures ANOVAs and one-way ANOVAs as appropriate, using Fisher’s least signficiant difference post hoc test. Differences were considered to be statistically significant at P<0.05.
Results
hemodynamic changes during coronary artery occlusion
In dogs subjected to a 25 min coronary artery occlusion (n=3 in both the control and PC groups), reductions in mean arterial blood pressure (approximately 10 mmHg to 15 mmHg), left ventricular systolic pressure (approximately 15 mmHg to 18 mmHg) and left ventricular positive and negative dP/dt (approximately 400 mmHg/s in the controls and approximately 240 mmHg/s in the preconditioned dogs) were observed. Left ventricular end-diastolic pressure increased from approximately 5 mmHg to 15 mmHg in control dogs, and to approximately 10 mmHg in the preconditioned animals. There was no substantial change in heart rate during occlusion in either of these groups.
The severity of ventricular arrhythmias and ischemia in dogs subjected to a 25 min occlusion of the LAd
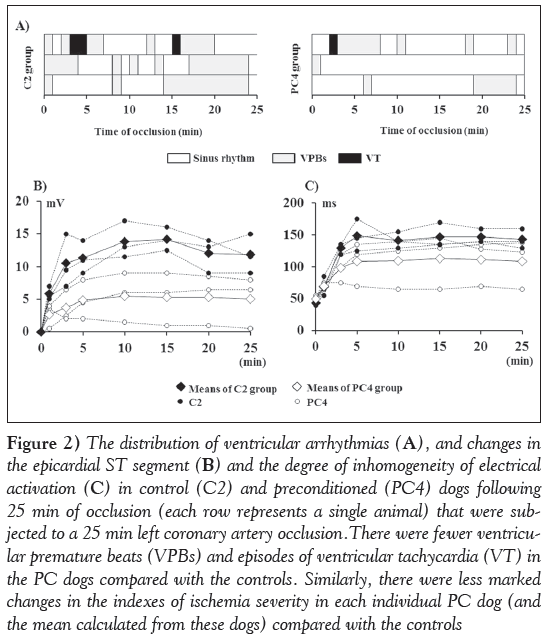
Figure 2 shows the distribution of ventricular arrhythmias (Figure 2A) and changes in the epicardial ST segment (Figure 2B) as well as in the degree of inhomogeneity (Figure 2C) in control (C2) and preconditioned (PC4) dogs that were subjected to a 25 min occlusion. The results show that the number of VPBs and the duration of the ectopic activity was lower in the preconditioned dogs compared with the controls; however, in both groups, only one of the three animals exhibited one or two short periods of ventricular tachycardia (Figure 2A). There were also less marked changes in the epicardial ST segment (Figure 2B) and in the degree of inhomogeneity of electrical activation (Figure 2C) in the preconditioned than in the control animals.
Figure 2: The distribution of ventricular arrhythmias (A), and changes in the epicardial ST segment (b) and the degree of inhomogeneity of electrical activation (C) in control (C2) and preconditioned (PC4) dogs following 25 min of occlusion (each row represents a single animal) that were subjected to a 25 min left coronary artery occlusion.There were fewer ventricular premature beats (VPBs) and episodes of ventricular tachycardia (VT) in the PC dogs compared with the controls. Similarly, there were less marked changes in the indexes of ischemia severity in each individual PC dog (and the mean calculated from these dogs) compared with the controls
Changes in nos activity following PC and prolonged coronary artery occlusion
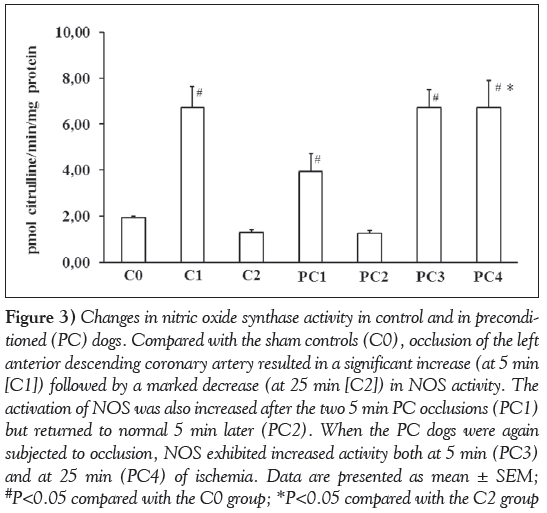
These are shown in Figure 3. Compared with the sham controls (C0), occlusion of the LAD for 5 min (C1) resulted in a significant increase in NOS enzyme activity. In contrast, the enzyme activity was markedly reduced in dogs that had been subjected to a 25 min occlusion (C2). Following the PC procedure (PC1), a marked increase in NOS activity was observed, but the activity of this enzyme returned to normal in samples obtained 5 min later (PC2). However, when the preconditioned dogs were subjected to ischemia again, in contrast to the controls, there were significant increases in NOS activity in samples taken both at 5 min (PC3) and 25 min (PC4) of the occlusion.
Figure 3: Changes in nitric oxide synthase activity in control and in preconditioned (PC) dogs. Compared with the sham controls (C0), occlusion of the left anterior descending coronary artery resulted in a significant increase (at 5 min [C1]) followed by a marked decrease (at 25 min [C2]) in NOS activity. The activation of NOS was also increased after the two 5 min PC occlusions (PC1) but returned to normal 5 min later (PC2). When the PC dogs were again subjected to occlusion, NOS exhibited increased activity both at 5 min (PC3) and at 25 min (PC4) of ischemia. Data are presented as mean ± SEM; #P<0.05 compared with the C0 group; *P<0.05 compared with the C2 group
Changes in plasma nox levels in control and in preconditioned dogs
These were almost parallel with changes in NOS activity (Figure 4). The baseline plasma level of NO metabolites in the blood of the coronary sinus was 20.3±0.1 μmol/L (data obtained at this time point [C0] from control and preconditioned dogs were combined). In control dogs, occlusion of the LAD resulted in a significant increase in NOx that was observed in blood samples collected at 7 min of ischemia (C1). Subsequently, the concentration of the NO metabolites began to decline and, by the end of the 25 min occlusion (C2), it was significantly lower than the initial baseline value. In contrast, the PC procedure significantly increased NOx (PC1 and PC2), and these values were maintained or even further increased during the subsequent 25 min occlusion (PC3 and PC4).
Figure 4: Changes in plasma nitrate/nitrite (NOx) levels of the blood of coronary sinus in control (C) and in preconditioned (PC) dogs. In control dogs subjected to a 25 min occlusion, the level of NO metabolites, after an initial increase (which peaked at approximately 7 min of ischaemia), was markedly decreased. In contrast to this, in PC dogs, the NOx levels were significantly increased both after the PC procedure and throughout the entire prolonged period of the occlusion. Data presented as mean ± SEM; #P<0.05 compared with the initial baseline value; *P<0.05 compared with the C2 group
Myocardial tissue superoxide production in control and in preconditioned dogs
These are shown in Figure 5. Compared with the sham controls, occlusion of the LAD for 25 min significantly increased the superoxide production (C2), indicating that a substantial amount of reactive oxygen species (ROS) can be generated already during ischemia. Conversely, the production of superoxide during the prolonged occlusion in the PC dogs was significantly less pronounced than in the controls (C2 compared with PC4; P<0.05), although the PC procedure itself increased ROS generation (PC1 and PC2).
Figure 5: Changes in myocardial superoxide production in control (C) and in preconditioned (PC) dogs. Compared with the sham control (C0) group, occlusion of the left anterior descending coronary artery increased the generation of superoxide, which was particularly clear by the end of the 25 min of ischemia. The superoxide production was also significantly increased during the brief PC occlusion and reperfusions insults, but, compared with the controls, when these dogs were subjected to a subsequent short (5 min; PC3) or prolonged (25 min; PC4) period of ischemia there was a reduction rather than an increase in the generation of superoxide. Data presented as mean ± SEM; #P<0.05 compared with C0; *P<0.05 compared with the C2 group (exposed to 25 min occlusion)
Changes in nt formation in control and in preconditioned dogs
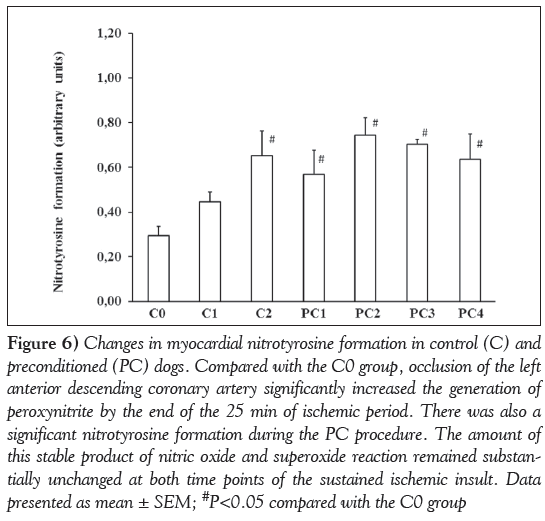
To assess peroxynitrite formation, NT production was determined in tissue samples taken from control and preconditioned dogs at various time points of the experiment (Figure 6). Compared with the sham controls, NT formation was significantly increased in dogs subjected to ischemia (C0 compared with C2; P<0.05). There was also enhanced NT production following the PC procedure (C0 compared with PC1 and PC2; P<0.05); however, the amount of this stable product of the reaction between NO and superoxide was not substantially changed during the subsequent, more prolonged period of occlusion (PC2 compared with PC3 and PC4), nor significantly different from that obtained in the controls (C2 compared with PC4).
Figure 6: Changes in myocardial nitrotyrosine formation in control (C) and preconditioned (PC) dogs. Compared with the C0 group, occlusion of the left anterior descending coronary artery significantly increased the generation of peroxynitrite by the end of the 25 min of ischemic period. There was also a significant nitrotyrosine formation during the PC procedure. The amount of this stable product of nitric oxide and superoxide reaction remained substantially unchanged at both time points of the sustained ischemic insult. Data presented as mean ± SEM; #P<0.05 compared with the C0 group
Discussion
The aim of the present study was to examine whether changes occurring during coronary artery occlusion in the plasma concentrations of NO metabolites (NOx) in anesthetized dogs were due to alterations in NOS activity and function. This question was raised because the results of one of our earlier studies [2] showed that in dogs subjected to a 25 min coronary artery occlusion, the plasma concentration of NO metabolites in the coronary sinus blood exhibit a biphasic change; ie, after an initial and transient elevation that occurs at approximately 7 min of ischemia, it begins to markedly decline, and by the end of the occlusion period the level of NOx is significantly less than the initial value [2]. This study also demonstrated that PC elevates NOx and prevents the reduction in NO bioavailability during a more prolonged occlusion – an effect to which we attribute importance in the antiarrhythmic effect of PC [2]. Because the NOS enzyme function was not determined in this particular study, we designed experiments in which myocardial NOS activity, superoxide and NT productions, as well as plasma NOx levels, were simultaneously assessed in samples collected from control and PC dogs at various time points during the experiments.
We have found that NOS activity was substantially increased after a 5 min period of coronary artery occlusion (C1; Figure 3), which resulted in an immediate elevation in the concentrations of NO metabolites in the coronary sinus blood (C1; Figure 4). This finding is consistent with previous observations that ischemia rapidly activates NOS [14] as a result of changes in the cytosolic calcium concentration and the phosphorylation of NOS [15]. However, if the occlusion was maintained over a longer period (eg, 25 min), the activity of NOS and, simultaneously, the NOx levels were significantly decreased (C2; Figures 3 and 4). It may well be that this reduction in NOx during prolonged occlusion is not simply a result of a loss of NOS function, but also from a reaction of NO with superoxide to form peroxynitrite. Indeed, in samples obtained at the end of the 25 min occlusion period, there were marked increases in the superoxide (C2; Figure 5) and the NT (C2; Figure 6) productions. This is also consistent with the previous experience that superoxide can also be generated during ischemia [16,17], particularly when the NOS enzyme uncouples because of the limited supply of oxygen, substrate and cofactors. This would lead to decreased NO and, in parallel, increased superoxide production by NOS [18,19].
The present study has also found that PC stimulates NOS; but interestingly the activity of NOS following the two 5 min PC occlusions (PC1 group) was not as marked as that could be observed after a single 5 min occlusion (C1 group). An explanation for this difference in NOS activity between these groups may lie in the reperfusion interval which elapses between the first and the second occlusion in the PC1 dogs, suggesting that the NOS enzyme is not only rapidly activated by ischemia [14], but it can also rapidly be deactivated when the ischemia was ceased. This assumption is confirmed by the results obtained from samples of PC2 dogs, which clearly show that NOS activity returned to the normal value after 5 min of the release of the second PC occlusion. Interestingly, despite this decrease in NOS activation, the plasma NOx levels appeared to be maintained over the entire PC procedure. There was also an apparent increase in superoxide and NT production during the consecutive 5 min periods of PC occlusion and reperfusion insults, supporting the role of these nitrogen and oxygen radicals in the initiation of the PC-induced protection [1,20-22]. However, we assume that these radicals generated during the PC procedure may also have a regulatory effect on NOS activity, whereby they contribute to the normalization of NOS activity soon after the release of the second PC occlusion. Indeed, studies examining endothelial NOS activity and NO bioavailability in relation to normal and impaired vascular function [15,23-26] have revealed that the ischemia and reperfusion-induced free radical products are able to modify NOS activity by acting on its various regulatory domains to maintain the balance between NO and superoxide production [27,28]. For example, it has been shown that an excess in superoxide production would deactivate NOS, resulting in reduced free NO levels and subsequent peroxynitrite formation [29]. Similarly, under certain conditions, the excessively generated NO can regulate the subsequent enzymatic synthesis of NO through a negative feedback (autoinhibitory) mechanism [30-32]. Although whether these radicals are, indeed, involved in the regulation of NOS under the circumstances of our experiment is not known, it is clear that 5 min after the release of the second PC occlusion the activity of NOS has returned to the normal value, whereas the concentration of NO, superoxide and NT remains elevated.
When the preconditioned dogs were again subjected to occlusion (5 min or 25 min), the activity of NOS and, simultaneously, the NOx levels were rapidly increased (PC3 group); however, in contrast to the controls, these were maintained or even further increased by the end of the 25 min occlusion (Figures 3 and 4; C2 compared with PC4, P<0.05). Conversely, the superoxide production during the prolonged occlusion was substantially less marked in the preconditioned dogs than in the controls (Figure 5), whereas such a reduction in the NT production was not apparent (Figure 6). The reason for this may be that because NT is a stable product, the NT production determined at the end of the prolonged ischemia includes the previously formed peroxynitrite as well. Nevertheless, our results, obtained from the parallel measurement of NOS activation and products – which represents an advantage of the present study – clearly demonstrate that PC preserves the function of NOS and, as a result, increases NO bioavailability and decreases superoxide production during a subsequent prolonged period of occlusion. This finding confirms previous observations in isolated rat hearts that PC prevents the loss of NOS enzymes and enhances NOS activity during ischemia and reperfusion [33].
There is some evidence, mainly from in vitro studies, that phosphorylation of NOS at various sites can rapidly modify NOS activity [34]. For example, Yang et al [35] recently reported that PC, through the stimulation of the Akt-protein kinase A pathway, activates endothelial NOS via serine 1176 phosphorylation in isolated rat hearts. In addition, post-translational modifications, such as protein-protein interactions, have been suggested to be important mechanisms in NOS regulation [36]. Despite all attempts, the precise mechanism by which PC preserves the function of NOS has not yet been fully elucidated. However, it was not the objective of the present study to determine this; rather, the main focus of the present study was a systematic evaluation of NOS activation with a simultaneous measurement of its products during ischemia and following PC in an in vivo large animal model. Considering our data and those of others, we speculate that PC, perhaps by preventing the uncoupling of NOS during ischemia, may influence the function of the enzyme. It is well known that NOS primarily produces NO in the presence of adequate supply of substrate and cofactors such as tetrahydrobiopterin (BH4), NADPH, FAD and flavin mononucleotide [37]. Among these, BH4 appears to be an essential cofactor for NOS to produce NO [18] by stabilizing NOS dimers (reductase and oxygenase domains [38]), increasing substrate affinity to the enzyme [32] and inhibiting NOS-mediated superoxide production [18]. There is also evidence that the bioavailability of BH4 is reduced under ischemic conditions and this, rather than the inadequate substrate availability [28], results in uncoupling of NOS and leading to superoxide rather than NO production [39]. In fact, it has been found that in diseases associated with impaired endothelial function, such as hypertension and diabetes, supplementation with BH4 improved endothelial function [40]. Furthermore, in anesthetized rabbits, PC decreased infarct size and increased BH4 concentrations after a 30 min coronary artery occlusion [41], which suggests that by elevating BH4 levels, PC prevents the uncoupling of NOS and preserves the function of NOS to produce NO even during a prolonged ischemic period. Although the concentrations of BH4 were not determined in the present study, it is reasonable to hypothesize that a similar mechanism may play a role in our model. If so, this would certainly explain the observed increase in NOS activity and NO synthesis as well as the decrease in superoxide production in the PC group compared with the controls.
Summary
The results of the present study demonstrate that a short period of ischemia rapidly activates the NOS enzyme and results in increased NO generation in anesthetized dogs. In contrast, a longer period of ischemia decreases NOS activity and NO bioavailability, with a parallel increase in superoxide and peroxynitrite production. This effect may be associated with the uncoupling of NOS, although the formation of peroxynitrite may also play a role in the decrease of NO. The present study also showed that PC induced by brief occlusion and reperfusion insults enhances NOS activity and NO synthesis, but also elevates the production of ROS; all of these radicals are believed to play a trigger role in the PC-induced protection. Although we do not know how these radicals, generated during the PC procedure, modify NOS activity, it is clear that PC preserves the function of NOS during prolonged occlusion, and results in enhanced NO and suppressed superoxide production. We hypothesize that this mechanism plays an important role in the antiarrhythmic effect of PC in our model.
Acknowledgement
The authors are grateful to Dr József Kaszaki for his valuable assistance in the determination of NOx at the Institute of Surgical Research. They are also grateful to the excellent technical assistance of Erika Bakó and Irene Biczók.
Funding
This work was supported by the Hungarian Scientific Research Foundation (OTKA; Project number K105252), the Gedeon Richter Centenarium Foundation and by the European Union and the State of Hungary, co-financed by the European Social Fund in the framework of TÁMOP-4.2.4.A/ 2-11/1-2012-0001 ‘National Excellence Program’.
Disclosures
The authors have no financial disclosures or conflicts of interest to declare.
References
- Végh Á, Szekeres L, Parratt J. Preconditioning of the ischaemic myocardium; involvement of the L-arginine nitric oxide pathway. Br J Pharmacol 1992;107:648-52.
- Kiss A, Juhász L, Seprényi G, Kupai K, Kaszaki J, Végh Á. The role of nitric oxide, superoxide and peroxynitrite in the anti-arrhythmic effects of preconditioning and peroxynitrite infusion in anaesthetized dogs. Br J Pharmacol 2010;160:1263-72.
- Zweier JL, Wang P, Kuppusamy P. Direct measurement of nitric oxide generation in the ischemic heart using electron paramagnetic resonance spectroscopy. J Biol Chem 1995;270:304-7.
- Zweier JL, Wang P, Samouilov A, Kuppusamy P. Enzyme-independent formation of nitric oxide in biological tissues. Nat Med 1995;1:804-9.
- Csonka C, Szilvássy Z, Fülöp F, et al. Classic preconditioning decreases the harmful accumulation of nitric oxide during ischemia and reperfusion in rat hearts. Circulation 1999;100:2260-6.
- Mori E, Haramaki N, Ikeda H, Imaizumi T. Intra-coronary administration of L-arginine aggravates myocardial stunning through production of peroxynitrite in dogs. Cardiovasc Res 1998;40:113-23.
- Stevens RM, Jahania MS, Stivers JE, Mentzer RM Jr, Lasley RD. Effects of in vivo myocardial ischemia and reperfusion on interstitial nitric oxide metabolites. Ann Thorac Surg 2002;4:1261-6.
- Prasan AM, McCarron HC, Zhang Y, Jeremy RW. Myocardial release of nitric oxide during ischaemia and reperfusion: Effects of L-arginine and hypercholesterolemia. Heart Lung Circ 2007;16:274-81.
- Végh Á, Komori S, Szekeres L, Parratt JR. Antiarrhythmic effects of preconditioning in anaesthetised dogs and rats. Cardiovasc Res 1992;26:487-95.
- Walker MJ, Curtis MJ, Hearse DJ, et al. The Lambeth Conventions: Guidelines for the study of arrhythmias in ischaemia, infarction, and reperfusion. Cardiovasc Res 1988;22:447-55.
- Kisvári G, Kovács M, Gardi J, Seprényi Gy, Kaszaki J, Végh Á. The effect of acute simvastatin administration on the severity of arrhythmias resulting from ischaemia and reperfusion in the canine: Is there a role for nitric oxide? Eur J Pharmacol 2014;732:96-104.
- Juhász L, Kiss A, Nyes? E, et al. Is there a trigger role of peroxynitrite in the anti-arrhythmic effect of ischaemic preconditioning and peroxynitrite infusion? Eur J Pharmacol 2011;667:306-13.
- Kiss A, Juhász L, Huliák I, Végh Á. Peroxynitrite decreases arrhythmias induced by ischaemia/reperfusion in anaesthetised dogs, without involving mitochondrial KATP channels. Br J Pharmacol 2008;155:1015-24.
- Depré C, Fiérain L, Hue L. Activation of nitric oxide synthase by ischaemia in the perfused heart. Cardiovasc Res 1997;33:82-7.
- Govers R, Rabelink TJ. Cellular regulation of endothelial nitric oxide synthase. Am J Physiol Renal Physiol 2001;280:F193-206.
- Vanden Hoek TL, Li C, Shao Z, Schumacker PT, Becker LB. Significant levels of oxidants are generated by isolated cardiomyocytes during ischemia prior to reperfusion. J Mol Cell Cardiol 1997;29:2571-83.
- Becker LB, Vanden Hoek TL, Shao ZH, Li CQ, Schumacker PT. Generation of superoxide in cardiomyocytes during ischemia before reperfusion. Am J Physiol 1999;277:H2240-6.
- Xia Y, Tsai AL, Berka V, Zweier JL. Superoxide generation from endothelial nitric-oxide synthase. J Biol Chem 1998;273:25804-8.
- Dumitrescu C, Biondi R, Xia Y, et al. Myocardial ischemia results in tetrahydrobiopterin (BH4) oxidation with impaired endothelial function ameliorated by BH4. Proc Natl Acad Sci 2007;104:15081-6.
- Tanaka M, Fujiwara H, Yamasaki K, Sasayama S. Superoxide dismutase and n-2-mercaptopropionyl glycine attenuate infarct size limitation effect of ischaemic preconditioning in the rabbit. Cardiovasc Res 1994;28:980-6.
- Pain T, Yang X-M, Critz SD, et al. Opening of mitochondrial KATP channels triggers the preconditioned state by generating free radicals. Circ Res 2000;87:460-6.
- Ferdinandy P, Schulz R. Nitric oxide, superoxide, and peroxynitrite in myocardial ischaemia-reperfusion injury and preconditioning. Br J Pharmacol 2003;138:532-43.
- Harrison DG. Perspective series: Nitric oxide and nitric oxide synthases. Cellular and molecular mechanisms of endothelial cell dysfunction. J Clin Invest 1997;100:2153-7.
- Peterson TE, Poppa V, Ueba H, Wu A, Yan C, Berk BC. Opposing effects of reactive oxygen species and cholesterol on endothelial nitric oxide synthase and endothelial cell caveolae. Circ Res 1999;85:29-37.
- Guzik TJ, Mussa S, Gastaldi D, et al. Mechanisms of increased vascular superoxide production in human diabetes mellitus: Role of NAD(P)H oxidase and endothelial nitric oxide synthase. Circulation 2002;105:1656-62.
- Chen CA, Druhan LJ, Varadharaj S, Chen YR, Zweier JL. Phosphorylation of endothelial nitric oxide synthase regulates superoxide generation from the enzyme. J Biol Chem 2008;283:27038-47.
- Stuehr DJ, Santolini J, Wang Z-Q, Wei C-C, Adak S. Update on mechanism and catalytic regulation in the NO synthases. J Biol Chem 2004;279:36167-70.
- Chatterjee A, Catravas JD. Endothelial nitric oxide (NO) and its pathologic regulation. Vascul Pharmacol 2008;49:134-40.
- Yaqoob M, Edelstein CL, Schrier RW. Role of nitric oxide and superoxide balance in hypoxia-reoxygenation proximal tubular injury. Nephrol Dial Transplant 1996;11:1743-6.
- Rengasamy A, Johns RA. Regulation of nitric oxide synthase by nitric oxide. Mol Pharmacol 1993;44:124-8.
- Kotsonis P, Frey A, Fröhlich LG, et al. Autoinhibition of neuronal nitric oxide synthase: Distinct effects of reactive nitrogen and oxygen species on enzyme activity. Biochem J 1999;340:745-52.
- Kotsonis P, Fröhlich LG, Shutenko ZV, Horejsi R, Pfleiderer W, Schmidt HH. Allosteric regulation of neuronal nitric oxide synthase by tetrahydrobiopterin and suppression of auto-damaging superoxide. Biochem J 2000;346:767-76.
- Muscari C, Bonafe F, Gamberini C, et al. Early preconditioning prevents the loss of endothelial nitric oxide synthase and enhances its activity in the ischemic/reperfused rat heart. Life Sci 2004;74:1127-37.
- Mount PF, Kemp BE, Power DA. Regulation of endothelial and myocardial NO synthesis by multi-site eNOS phosphorylation. J Mol Cell Cardiol 2007;42:271-9.
- Yang C, Talukder MA, Varadharaj S, Velayutham M, Zweier JL. Early ischaemic preconditioning requires Akt- and PKA-mediated activation of eNOS via serine 1176 phosporylation. Cardiovasc Res 2013;97:33-43.
- Gratton JP, Fontana J, O’Connor DS, Garcia-Gardena G, McCabe TJ, Sessa WC. Reconstitution of an endothelial nitric-oxide synthase (eNOS), hsp90, and caveolin-1 complex in vitro. Evidence that hsp90 facilitates calmodulin stimulated displacement of eNOS from caveolin-1. J Biol Chem 2000;275:22268-72.
- Cosentino F, Lüscher TF. Tetrahydrobiopterin and endothelial nitric oxide synthase activity. Cardiovasc Res 1999;43:274-8.
- Moens AL, Kass DA. Tetrahydrobiopterin and cardiovascular disease. Arterioscler Thromb Vasc Biol 2006;26:2439-44.
- Vasquez-Vivar J, Kalyanaraman B, Martasek P, et al. Superoxide generation by endothelial nitric oxide synthase: The influence of cofactors. Proc Natl Acad Sci USA 1998;95:9220-5.
- Heitzer T, Brockhoff C, Mayer B, et al. Tetrahydrobiopterin improves endothelium-dependent vasodilatation in chronic smokers: Evidence for a dysfunctional nitric oxide synthase. Circ Res 2000;86:E36.
- Vladic N, Ge Z-D, Leucker T, et al. Decreased tetrahydrobiopterin and disrupted association of Hsp90 with eNOS by hyperglycaemia impair myocardial ischemic preconditioning. Am J Physiol Heart Circ Physiol 2011;301:H2130-9.
- *Corresponding Author:
- Prof Dr Ágnes Végh
Department of Pharmacology and Pharmacotherapy, University of Szeged, Albert Szent-Györgyi Faculty of Medicine, Dóm tér 12, PO Box 427, H-6720 Hungary.
Tel:36-62-545-673
Fax:36-63-545-680
E-mail:vegh.agnes@med.u-szeged.hu
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact support@pulsus.com
Abstract
obJeCtives: To examine whether changes in nitric oxide (NO) bioavailability occurring during a 25 min occlusion of the left anterior descending coronary artery and following ischemic preconditioning (PC) in anesthetized dogs (chloralose and urethane, intravenous) are associated with changes in the activation of NO synthase (NOS) enzymes.
Methods: In 12 control and 16 preconditioned dogs, total NOS activity (measured using radioimmunoassay), superoxide (measured using confocal microscopy) and nitrotyrosine (NT; measured using Western blot) production were assessed in tissue samples obtained from the left ventricular wall both after the PC (two 5 min occlusions) procedure and at two time points (5 min and 25 min) during prolonged ischemia. Plasma nitrate/nitrite concentrations in the blood of the coronary sinus were also determined.
ResuLts: Compared with the sham control group that was not subjected to ischemia, occlusion of the left anterior descending coronary artery resulted in an initial increase, followed by a significant decrease in NOS activity and nitrate/nitrite levels. There were parallel increases in superoxide and NT production by the end of the ischemic period. PC itself enhanced NOS activity and the concentration of NO metabolites, which were maintained during the prolonged occlusion. Furthermore, PC prevented prolonged ischemia-induced superoxide and NT formation.
ConCLusions: The present study demonstrated that changes in NO bioavailability during ischemia are closely associated with changes in NOS activation. PC stimulates and maintains the activation of NOS, thereby providing better NO bioavailability and suppressed free radical formation during sustained ischemia. All of these effects may have importance in the previously observed marked antiarrhythmic effect of PC.
-Keywords
Ischemia/reperfusion; Nitrate/nitrite; NOS activity; Preconditioning; Superoxide
The first evidence that nitric oxide (NO) plays an essential role in the cardioprotective effects of preconditioning (PC) resulted from studies involving anesthetized dogs, which demonstrated that the marked antiarrhythmic effect of PC, induced by brief periods of coronary artery occlusion, were abolished when L-NG-nitroarginine methyl ester, an inhibitor of NO formation, was administered either before the PC procedure or immediately before a prolonged (25 min) ischemic insult [1]. These results also suggested that NO may play both a trigger and mediator role in this antiarrhythmic protection. More recently, we have provided further evidence of this by measuring the plasma concentrations of NO metabolites (nitrate and nitrite, NOx) in the blood of the coronary sinus [2]. The results showed that PC elevated NOx levels, and these were maintained or even further increased during prolonged occlusion, in contrast to the nonpreconditioned controls, in which NOx, after an initial increase, was substantially reduced [2].
There remains ongoing debate regarding whether the generation of NO increases or decreases during myocardial ischemia, and whether the protection provided by PC is due to a reduction or an elevation of NO bioavailability. A possible explanation for these controversies may be found in the differences in the experimental methods and in the methods used for NO detection. For example, the results of studies (mainly in vitro studies) using electron paramagnetic resonance spectroscopy for NO detection suggest that the ischemic milieu (low pH, reduced oxygen availability, etc) facilitates the conversion of nitrite to NO, resulting in enhanced formation and accumulation of nonenzymatic NO, which reacts with superoxide to form toxic peroxynitrite [3-5]. Therefore, by attenuating the excess of NO production and subsequent peroxynitrite formation, PC would result in cardioprotection [5]. These findings were, however, not confirmed by the results of the in vivo experiments. Studies involving anesthetized dogs and pigs using methods other than electron paramagnetic resonance spectroscopy (eg, microdialysis and electrochemical techniques) for NO detection indicated that NO production is decreased rather than increased during sustained coronary artery occlusion [6,7]. For example, it has been found that in anesthetized pigs subjected to a 15 min period of occlusion, the release of NO in the interstitial fluid was markedly reduced [7]. Moreover, in isolated rabbit hearts, the release of NO in the coronary sinus was significantly decreased after 60 min of ischemia [8]. Our results in anesthetized dogs have confirmed these findings, but we have also noted that the decrease in NO bioavailability during prolonged ischemia is always preceded by an initial increase in NOx that occurs soon (approximately 7 min) after the onset of the occlusion [2]. We have also reported that the NOx levels are significantly elevated by the brief periods of PC occlusion, and that these levels are maintained or even further increased during subsequent, more prolonged ischemia. We hypothesize that the increased NO bioavailability during ischemia may be important for the antiarrhythmic effect of PC [2].
Because nitric oxide synthase (NOS) activity was not measured in the above-mentioned study, we have designed experiments to examine, using a limited number of dogs, whether changes in NO metabolites during coronary artery occlusion in preconditioned and nonpreconditioned dogs are due to alterations in NOS activity. In parallel, we also determined superoxide and nitrotyrosine (NT) production.
Methods
Ethics statement
The origin and maintenance of the dogs used in the present study were in accordance with Hungarian law (XXVIII, chapter IV, paragraph 31) regarding large experimental animals, which conforms to the Guide for the Care and Use of Laboratory Animals published by the United States National Institutes of Health (NIH Publication No. 85-23, revised 1996), and conformed to the Directive 2010/63/EU of the European Parliament. The protocols were approved by the Ethical Committee for the Protection of Animals in Research of the University of Szeged, Szeged, Hungary (approval number: I-74-5-2012) and by the Department of Animal Health and Food Control of the Ministry of Agriculture and Rural Development (authority approval number XIII/1211/2012).
Surgical preparation
Inbred mongrel dogs of either sex (body weight 16 kg to 20 kg) were used. Before the studies, the dogs were housed in an animal room (temperature 10°C to 20°C; humidity 40% to 70%; lighting 12 h per day; two animals per pen) for at least two weeks and fed a standard diet with ad libitum access to water. Food was withdrawn 24 h before anesthesia. Animals were anesthetized with a mixture of chloralose and urethane (60 mg/kg and 200 mg/kg intravenously, respectively; Sigma, USA), and ventilated with room air using a Harvard respirator at a rate and volume sufficient to maintain arterial blood gases and pH within normal limits [9]. The anesthesia was maintained by the administration of an additional slow injection of the same anesthetics (dose volume of 0.5 mL/kg). Body temperature was recorded at the midesophagus and maintained at 37±0.5°C by a heating pad.
The surgical procedures were similar to those described previously [9]. Briefly, polyethylene catheters were inserted into the right femoral artery for monitoring arterial blood pressure, and into the left ventricle for the measurement of systolic and end-diastolic pressures, and left ventricular dP/dt. The right femoral vein was also catheterized for further administration of anesthetic. Another catheter was positioned in the coronary sinus through the jugular vein, from which blood samples were collected for the measurement of plasma NOx levels.
A thoracotomy was performed at the fifth intercostal space and the left anterior descending artery (LAD) was prepared for occlusion just proximal to the first main diagonal branch. The severity of myocardial ischemia was assessed by changes in epicardial ST segment (mV) and in the degree of inhomogeneity of electrical activation (ms) recorded from the left ventricular wall distal to the occlusion site using a composite electrode, as previously described [9].
Ventricular arrhythmias during a 25 min period of occlusion were analyzed according to the ‘Lambeth Conventions’ [10] with the modification outlined previously [9]. Briefly, the total number of ventricular premature beats (VPBs), the incidence and the number of episodes of ventricular tachycardia (defined as a run of ≥4 VPBs at a rate faster than the resting heart rate), and the incidence of ventricular fibrillation were measured.
All parameters, together with a chest lead electrocardiogram, were monitored using a Plugsys Haemodynamic apparatus (Hugo Sachs Electronics, Germany) and recorded on a Graphtec Thermal Array Recorder (Hugo Sachs Electronics, Germany).
Assessment of plasma nitrate/nitrite levels
Plasma NOx concentrations were determined using the Griess reaction in blood samples collected from the coronary sinus at various time intervals (Figure 1) as described previously [2]. After sample preparation (centrifugation and enzymatic reduction of nitrate to nitrite), the Griess reagent was added to the mixture and incubated for an additional 10 min at room temperature. The absorbance of the azo compound was measured spectrophotometrically at a wavelength of 540 nm and the total NOx concentration (μmol/L) was determined using a standard calibration curve of NaNO2 and NaNO3 (Sigma, USA).
Figure 1: Outline of the experimental protocol. In three groups of control (C) dogs, myocardial tissue and blood samples (indicated by arrows) were collected either immediately after the euthanasia (C0; n=5) or 5 min (C1; n=4) as well as 25 min (C2; n=3) of the occlusion of the left anterior descending (LAD) coronary artery. An additional four groups of animals were subjected to preconditioning (PC) by two 5 min LAD occlusions interspersed with a 5 min interval of reperfusion. From these dogs, tissue and blood samples were obtained at the end of the second 5 min PC occlusion (PC1=5), at the end of the second 5 min reperfusion interval (PC2; n=4) as well as 5 min (PC3; n=4) and 25 min (PC4; n=3) of the prolonged ischemia. Myocardial nitric oxide synthase activity, superoxide and nitrotyrosine production in the tissue samples were determined. Plasma nitrate/nitrite levels were assessed in the blood samples withdrawn from the coronary sinus
Determination of nos activity
NOS activity was measuring using an assay kit (Cayman Chemical, USA) based on the biochemical conversion of [3H] L-arginine to [3H] L-citrulline by NOS, as has been described recently [11]. Briefly, according to the manufacturer’s instructions, total NOS protein was isolated from the myocardial tissue (100 mg), homogenized in ice-cold homogenization buffer (Cayman Chemical, USA) and centrifuged at 2000 g for 15 min. A liquid scintillation counter (Wizard, USA) was used to determine NOS activation by measuring the amount of radiolabelled citrulline. The activity of the enzyme was expressed as the percentage of total counts, corrected using the background counts per minute.
Assessment of myocardial superoxide production
Myocardial tissue blocks (0.5 cm × 0.5 cm × 2.0 cm) were excised from the ischemic LAD region and, after cryosectioning, the samples were stained with dihydroethidium (1 μmol/L, Sigma, USA) as described previously (2,12). Ten to 15 images were obtained from both from the stained samples and the negative controls (incubated with 100 mmol/L N-acetyl-L-cysteine, Sigma, USA) using a confocal microscope (Olympus FV1000, Japan). The intensity of the fluorescent signals was analysed using ImageJ software (National Institutes of Health, USA) and expressed in arbitrary units.
Determination of peroxynitrite production
To assess peroxynitrite production, the formation of NT was measured by Western blot, as previously described [13]. Briefly, after tissue preparation (homogenization and centrifugation), the protein concentration in the supernatant was determined using the method described by Lowry. Following electrophoresis, the proteins were transferred onto polyvinylidene fluoride membrane. After incubation with a monoclonal anti-NT antibody (MAB5404, Chemicon, USA) and horseradish peroxidase-conjugated rabbit anti-mouse immunoglobulin G (P0161, Dakocytomation, Denmark) as the secondary antibody, the membrane was developed using an enhanced chemiluminescence kit (ECL Plus, GE Healthcare, United Kingdom), exposed to x-ray film and scanned. ImageJ was used to determine the density of the NT bands. Equal loading was ensured using Coomassie Blue staining.
Experimental protocol
The experimental protocol is illustrated in Figure 1. The dogs were randomly assigned to one of seven groups, each containing between three and five animals. After surgery, the dogs were allowed to recover for 30 min. In all groups except the sham-operated controls, myocardial ischemia was induced by the occlusion of the LAD. Three groups served as controls. In these groups, after euthanasia by anesthetic overdose, myocardial tissue samples were harvested either at the end of the 30 min recovery period (C0; n=5) or at 5 min (C1; n=4) and 25 min (C2; n=3) of the coronary artery occlusion. In four groups, the dogs were preconditioned using two 5 min occlusions of the LAD, interspersed with a 5 min interval of reperfusion. In these groups, after euthanasia by anesthetic overdose, samples were taken at the end of the second PC occlusion (PC1; n=5), after the PC procedure but just before the 25 min occlusion (PC2; n=4), as well as at 5 min (PC3; n=4) and 25 min (PC4; n=3) of the prolonged ischemia. In each group, tissue samples to be used for the determination of NOS activity and superoxide production were excised from the ischemic area supplied by the occluded LAD. Blood samples were also collected from the coronary sinus at various time points of the experiment for the measurement of NOx levels.
Statistical analysis
Values are expressed as mean ± SEM, and the differences among means were compared using repeated-measures ANOVAs and one-way ANOVAs as appropriate, using Fisher’s least signficiant difference post hoc test. Differences were considered to be statistically significant at P<0.05.
Results
hemodynamic changes during coronary artery occlusion
In dogs subjected to a 25 min coronary artery occlusion (n=3 in both the control and PC groups), reductions in mean arterial blood pressure (approximately 10 mmHg to 15 mmHg), left ventricular systolic pressure (approximately 15 mmHg to 18 mmHg) and left ventricular positive and negative dP/dt (approximately 400 mmHg/s in the controls and approximately 240 mmHg/s in the preconditioned dogs) were observed. Left ventricular end-diastolic pressure increased from approximately 5 mmHg to 15 mmHg in control dogs, and to approximately 10 mmHg in the preconditioned animals. There was no substantial change in heart rate during occlusion in either of these groups.
The severity of ventricular arrhythmias and ischemia in dogs subjected to a 25 min occlusion of the LAd
Figure 2 shows the distribution of ventricular arrhythmias (Figure 2A) and changes in the epicardial ST segment (Figure 2B) as well as in the degree of inhomogeneity (Figure 2C) in control (C2) and preconditioned (PC4) dogs that were subjected to a 25 min occlusion. The results show that the number of VPBs and the duration of the ectopic activity was lower in the preconditioned dogs compared with the controls; however, in both groups, only one of the three animals exhibited one or two short periods of ventricular tachycardia (Figure 2A). There were also less marked changes in the epicardial ST segment (Figure 2B) and in the degree of inhomogeneity of electrical activation (Figure 2C) in the preconditioned than in the control animals.
Figure 2: The distribution of ventricular arrhythmias (A), and changes in the epicardial ST segment (b) and the degree of inhomogeneity of electrical activation (C) in control (C2) and preconditioned (PC4) dogs following 25 min of occlusion (each row represents a single animal) that were subjected to a 25 min left coronary artery occlusion.There were fewer ventricular premature beats (VPBs) and episodes of ventricular tachycardia (VT) in the PC dogs compared with the controls. Similarly, there were less marked changes in the indexes of ischemia severity in each individual PC dog (and the mean calculated from these dogs) compared with the controls
Changes in nos activity following PC and prolonged coronary artery occlusion
These are shown in Figure 3. Compared with the sham controls (C0), occlusion of the LAD for 5 min (C1) resulted in a significant increase in NOS enzyme activity. In contrast, the enzyme activity was markedly reduced in dogs that had been subjected to a 25 min occlusion (C2). Following the PC procedure (PC1), a marked increase in NOS activity was observed, but the activity of this enzyme returned to normal in samples obtained 5 min later (PC2). However, when the preconditioned dogs were subjected to ischemia again, in contrast to the controls, there were significant increases in NOS activity in samples taken both at 5 min (PC3) and 25 min (PC4) of the occlusion.
Figure 3: Changes in nitric oxide synthase activity in control and in preconditioned (PC) dogs. Compared with the sham controls (C0), occlusion of the left anterior descending coronary artery resulted in a significant increase (at 5 min [C1]) followed by a marked decrease (at 25 min [C2]) in NOS activity. The activation of NOS was also increased after the two 5 min PC occlusions (PC1) but returned to normal 5 min later (PC2). When the PC dogs were again subjected to occlusion, NOS exhibited increased activity both at 5 min (PC3) and at 25 min (PC4) of ischemia. Data are presented as mean ± SEM; #P<0.05 compared with the C0 group; *P<0.05 compared with the C2 group
Changes in plasma nox levels in control and in preconditioned dogs
These were almost parallel with changes in NOS activity (Figure 4). The baseline plasma level of NO metabolites in the blood of the coronary sinus was 20.3±0.1 μmol/L (data obtained at this time point [C0] from control and preconditioned dogs were combined). In control dogs, occlusion of the LAD resulted in a significant increase in NOx that was observed in blood samples collected at 7 min of ischemia (C1). Subsequently, the concentration of the NO metabolites began to decline and, by the end of the 25 min occlusion (C2), it was significantly lower than the initial baseline value. In contrast, the PC procedure significantly increased NOx (PC1 and PC2), and these values were maintained or even further increased during the subsequent 25 min occlusion (PC3 and PC4).
Figure 4: Changes in plasma nitrate/nitrite (NOx) levels of the blood of coronary sinus in control (C) and in preconditioned (PC) dogs. In control dogs subjected to a 25 min occlusion, the level of NO metabolites, after an initial increase (which peaked at approximately 7 min of ischaemia), was markedly decreased. In contrast to this, in PC dogs, the NOx levels were significantly increased both after the PC procedure and throughout the entire prolonged period of the occlusion. Data presented as mean ± SEM; #P<0.05 compared with the initial baseline value; *P<0.05 compared with the C2 group
Myocardial tissue superoxide production in control and in preconditioned dogs
These are shown in Figure 5. Compared with the sham controls, occlusion of the LAD for 25 min significantly increased the superoxide production (C2), indicating that a substantial amount of reactive oxygen species (ROS) can be generated already during ischemia. Conversely, the production of superoxide during the prolonged occlusion in the PC dogs was significantly less pronounced than in the controls (C2 compared with PC4; P<0.05), although the PC procedure itself increased ROS generation (PC1 and PC2).
Figure 5: Changes in myocardial superoxide production in control (C) and in preconditioned (PC) dogs. Compared with the sham control (C0) group, occlusion of the left anterior descending coronary artery increased the generation of superoxide, which was particularly clear by the end of the 25 min of ischemia. The superoxide production was also significantly increased during the brief PC occlusion and reperfusions insults, but, compared with the controls, when these dogs were subjected to a subsequent short (5 min; PC3) or prolonged (25 min; PC4) period of ischemia there was a reduction rather than an increase in the generation of superoxide. Data presented as mean ± SEM; #P<0.05 compared with C0; *P<0.05 compared with the C2 group (exposed to 25 min occlusion)
Changes in nt formation in control and in preconditioned dogs
To assess peroxynitrite formation, NT production was determined in tissue samples taken from control and preconditioned dogs at various time points of the experiment (Figure 6). Compared with the sham controls, NT formation was significantly increased in dogs subjected to ischemia (C0 compared with C2; P<0.05). There was also enhanced NT production following the PC procedure (C0 compared with PC1 and PC2; P<0.05); however, the amount of this stable product of the reaction between NO and superoxide was not substantially changed during the subsequent, more prolonged period of occlusion (PC2 compared with PC3 and PC4), nor significantly different from that obtained in the controls (C2 compared with PC4).
Figure 6: Changes in myocardial nitrotyrosine formation in control (C) and preconditioned (PC) dogs. Compared with the C0 group, occlusion of the left anterior descending coronary artery significantly increased the generation of peroxynitrite by the end of the 25 min of ischemic period. There was also a significant nitrotyrosine formation during the PC procedure. The amount of this stable product of nitric oxide and superoxide reaction remained substantially unchanged at both time points of the sustained ischemic insult. Data presented as mean ± SEM; #P<0.05 compared with the C0 group
Discussion
The aim of the present study was to examine whether changes occurring during coronary artery occlusion in the plasma concentrations of NO metabolites (NOx) in anesthetized dogs were due to alterations in NOS activity and function. This question was raised because the results of one of our earlier studies [2] showed that in dogs subjected to a 25 min coronary artery occlusion, the plasma concentration of NO metabolites in the coronary sinus blood exhibit a biphasic change; ie, after an initial and transient elevation that occurs at approximately 7 min of ischemia, it begins to markedly decline, and by the end of the occlusion period the level of NOx is significantly less than the initial value [2]. This study also demonstrated that PC elevates NOx and prevents the reduction in NO bioavailability during a more prolonged occlusion – an effect to which we attribute importance in the antiarrhythmic effect of PC [2]. Because the NOS enzyme function was not determined in this particular study, we designed experiments in which myocardial NOS activity, superoxide and NT productions, as well as plasma NOx levels, were simultaneously assessed in samples collected from control and PC dogs at various time points during the experiments.
We have found that NOS activity was substantially increased after a 5 min period of coronary artery occlusion (C1; Figure 3), which resulted in an immediate elevation in the concentrations of NO metabolites in the coronary sinus blood (C1; Figure 4). This finding is consistent with previous observations that ischemia rapidly activates NOS [14] as a result of changes in the cytosolic calcium concentration and the phosphorylation of NOS [15]. However, if the occlusion was maintained over a longer period (eg, 25 min), the activity of NOS and, simultaneously, the NOx levels were significantly decreased (C2; Figures 3 and 4). It may well be that this reduction in NOx during prolonged occlusion is not simply a result of a loss of NOS function, but also from a reaction of NO with superoxide to form peroxynitrite. Indeed, in samples obtained at the end of the 25 min occlusion period, there were marked increases in the superoxide (C2; Figure 5) and the NT (C2; Figure 6) productions. This is also consistent with the previous experience that superoxide can also be generated during ischemia [16,17], particularly when the NOS enzyme uncouples because of the limited supply of oxygen, substrate and cofactors. This would lead to decreased NO and, in parallel, increased superoxide production by NOS [18,19].
The present study has also found that PC stimulates NOS; but interestingly the activity of NOS following the two 5 min PC occlusions (PC1 group) was not as marked as that could be observed after a single 5 min occlusion (C1 group). An explanation for this difference in NOS activity between these groups may lie in the reperfusion interval which elapses between the first and the second occlusion in the PC1 dogs, suggesting that the NOS enzyme is not only rapidly activated by ischemia [14], but it can also rapidly be deactivated when the ischemia was ceased. This assumption is confirmed by the results obtained from samples of PC2 dogs, which clearly show that NOS activity returned to the normal value after 5 min of the release of the second PC occlusion. Interestingly, despite this decrease in NOS activation, the plasma NOx levels appeared to be maintained over the entire PC procedure. There was also an apparent increase in superoxide and NT production during the consecutive 5 min periods of PC occlusion and reperfusion insults, supporting the role of these nitrogen and oxygen radicals in the initiation of the PC-induced protection [1,20-22]. However, we assume that these radicals generated during the PC procedure may also have a regulatory effect on NOS activity, whereby they contribute to the normalization of NOS activity soon after the release of the second PC occlusion. Indeed, studies examining endothelial NOS activity and NO bioavailability in relation to normal and impaired vascular function [15,23-26] have revealed that the ischemia and reperfusion-induced free radical products are able to modify NOS activity by acting on its various regulatory domains to maintain the balance between NO and superoxide production [27,28]. For example, it has been shown that an excess in superoxide production would deactivate NOS, resulting in reduced free NO levels and subsequent peroxynitrite formation [29]. Similarly, under certain conditions, the excessively generated NO can regulate the subsequent enzymatic synthesis of NO through a negative feedback (autoinhibitory) mechanism [30-32]. Although whether these radicals are, indeed, involved in the regulation of NOS under the circumstances of our experiment is not known, it is clear that 5 min after the release of the second PC occlusion the activity of NOS has returned to the normal value, whereas the concentration of NO, superoxide and NT remains elevated.
When the preconditioned dogs were again subjected to occlusion (5 min or 25 min), the activity of NOS and, simultaneously, the NOx levels were rapidly increased (PC3 group); however, in contrast to the controls, these were maintained or even further increased by the end of the 25 min occlusion (Figures 3 and 4; C2 compared with PC4, P<0.05). Conversely, the superoxide production during the prolonged occlusion was substantially less marked in the preconditioned dogs than in the controls (Figure 5), whereas such a reduction in the NT production was not apparent (Figure 6). The reason for this may be that because NT is a stable product, the NT production determined at the end of the prolonged ischemia includes the previously formed peroxynitrite as well. Nevertheless, our results, obtained from the parallel measurement of NOS activation and products – which represents an advantage of the present study – clearly demonstrate that PC preserves the function of NOS and, as a result, increases NO bioavailability and decreases superoxide production during a subsequent prolonged period of occlusion. This finding confirms previous observations in isolated rat hearts that PC prevents the loss of NOS enzymes and enhances NOS activity during ischemia and reperfusion [33].
There is some evidence, mainly from in vitro studies, that phosphorylation of NOS at various sites can rapidly modify NOS activity [34]. For example, Yang et al [35] recently reported that PC, through the stimulation of the Akt-protein kinase A pathway, activates endothelial NOS via serine 1176 phosphorylation in isolated rat hearts. In addition, post-translational modifications, such as protein-protein interactions, have been suggested to be important mechanisms in NOS regulation [36]. Despite all attempts, the precise mechanism by which PC preserves the function of NOS has not yet been fully elucidated. However, it was not the objective of the present study to determine this; rather, the main focus of the present study was a systematic evaluation of NOS activation with a simultaneous measurement of its products during ischemia and following PC in an in vivo large animal model. Considering our data and those of others, we speculate that PC, perhaps by preventing the uncoupling of NOS during ischemia, may influence the function of the enzyme. It is well known that NOS primarily produces NO in the presence of adequate supply of substrate and cofactors such as tetrahydrobiopterin (BH4), NADPH, FAD and flavin mononucleotide [37]. Among these, BH4 appears to be an essential cofactor for NOS to produce NO [18] by stabilizing NOS dimers (reductase and oxygenase domains [38]), increasing substrate affinity to the enzyme [32] and inhibiting NOS-mediated superoxide production [18]. There is also evidence that the bioavailability of BH4 is reduced under ischemic conditions and this, rather than the inadequate substrate availability [28], results in uncoupling of NOS and leading to superoxide rather than NO production [39]. In fact, it has been found that in diseases associated with impaired endothelial function, such as hypertension and diabetes, supplementation with BH4 improved endothelial function [40]. Furthermore, in anesthetized rabbits, PC decreased infarct size and increased BH4 concentrations after a 30 min coronary artery occlusion [41], which suggests that by elevating BH4 levels, PC prevents the uncoupling of NOS and preserves the function of NOS to produce NO even during a prolonged ischemic period. Although the concentrations of BH4 were not determined in the present study, it is reasonable to hypothesize that a similar mechanism may play a role in our model. If so, this would certainly explain the observed increase in NOS activity and NO synthesis as well as the decrease in superoxide production in the PC group compared with the controls.
Summary
The results of the present study demonstrate that a short period of ischemia rapidly activates the NOS enzyme and results in increased NO generation in anesthetized dogs. In contrast, a longer period of ischemia decreases NOS activity and NO bioavailability, with a parallel increase in superoxide and peroxynitrite production. This effect may be associated with the uncoupling of NOS, although the formation of peroxynitrite may also play a role in the decrease of NO. The present study also showed that PC induced by brief occlusion and reperfusion insults enhances NOS activity and NO synthesis, but also elevates the production of ROS; all of these radicals are believed to play a trigger role in the PC-induced protection. Although we do not know how these radicals, generated during the PC procedure, modify NOS activity, it is clear that PC preserves the function of NOS during prolonged occlusion, and results in enhanced NO and suppressed superoxide production. We hypothesize that this mechanism plays an important role in the antiarrhythmic effect of PC in our model.
Acknowledgement
The authors are grateful to Dr József Kaszaki for his valuable assistance in the determination of NOx at the Institute of Surgical Research. They are also grateful to the excellent technical assistance of Erika Bakó and Irene Biczók.
Funding
This work was supported by the Hungarian Scientific Research Foundation (OTKA; Project number K105252), the Gedeon Richter Centenarium Foundation and by the European Union and the State of Hungary, co-financed by the European Social Fund in the framework of TÁMOP-4.2.4.A/ 2-11/1-2012-0001 ‘National Excellence Program’.
Disclosures
The authors have no financial disclosures or conflicts of interest to declare.
References
- Végh Á, Szekeres L, Parratt J. Preconditioning of the ischaemic myocardium; involvement of the L-arginine nitric oxide pathway. Br J Pharmacol 1992;107:648-52.
- Kiss A, Juhász L, Seprényi G, Kupai K, Kaszaki J, Végh Á. The role of nitric oxide, superoxide and peroxynitrite in the anti-arrhythmic effects of preconditioning and peroxynitrite infusion in anaesthetized dogs. Br J Pharmacol 2010;160:1263-72.
- Zweier JL, Wang P, Kuppusamy P. Direct measurement of nitric oxide generation in the ischemic heart using electron paramagnetic resonance spectroscopy. J Biol Chem 1995;270:304-7.
- Zweier JL, Wang P, Samouilov A, Kuppusamy P. Enzyme-independent formation of nitric oxide in biological tissues. Nat Med 1995;1:804-9.
- Csonka C, Szilvássy Z, Fülöp F, et al. Classic preconditioning decreases the harmful accumulation of nitric oxide during ischemia and reperfusion in rat hearts. Circulation 1999;100:2260-6.
- Mori E, Haramaki N, Ikeda H, Imaizumi T. Intra-coronary administration of L-arginine aggravates myocardial stunning through production of peroxynitrite in dogs. Cardiovasc Res 1998;40:113-23.
- Stevens RM, Jahania MS, Stivers JE, Mentzer RM Jr, Lasley RD. Effects of in vivo myocardial ischemia and reperfusion on interstitial nitric oxide metabolites. Ann Thorac Surg 2002;4:1261-6.
- Prasan AM, McCarron HC, Zhang Y, Jeremy RW. Myocardial release of nitric oxide during ischaemia and reperfusion: Effects of L-arginine and hypercholesterolemia. Heart Lung Circ 2007;16:274-81.
- Végh Á, Komori S, Szekeres L, Parratt JR. Antiarrhythmic effects of preconditioning in anaesthetised dogs and rats. Cardiovasc Res 1992;26:487-95.
- Walker MJ, Curtis MJ, Hearse DJ, et al. The Lambeth Conventions: Guidelines for the study of arrhythmias in ischaemia, infarction, and reperfusion. Cardiovasc Res 1988;22:447-55.
- Kisvári G, Kovács M, Gardi J, Seprényi Gy, Kaszaki J, Végh Á. The effect of acute simvastatin administration on the severity of arrhythmias resulting from ischaemia and reperfusion in the canine: Is there a role for nitric oxide? Eur J Pharmacol 2014;732:96-104.
- Juhász L, Kiss A, Nyes? E, et al. Is there a trigger role of peroxynitrite in the anti-arrhythmic effect of ischaemic preconditioning and peroxynitrite infusion? Eur J Pharmacol 2011;667:306-13.
- Kiss A, Juhász L, Huliák I, Végh Á. Peroxynitrite decreases arrhythmias induced by ischaemia/reperfusion in anaesthetised dogs, without involving mitochondrial KATP channels. Br J Pharmacol 2008;155:1015-24.
- Depré C, Fiérain L, Hue L. Activation of nitric oxide synthase by ischaemia in the perfused heart. Cardiovasc Res 1997;33:82-7.
- Govers R, Rabelink TJ. Cellular regulation of endothelial nitric oxide synthase. Am J Physiol Renal Physiol 2001;280:F193-206.
- Vanden Hoek TL, Li C, Shao Z, Schumacker PT, Becker LB. Significant levels of oxidants are generated by isolated cardiomyocytes during ischemia prior to reperfusion. J Mol Cell Cardiol 1997;29:2571-83.
- Becker LB, Vanden Hoek TL, Shao ZH, Li CQ, Schumacker PT. Generation of superoxide in cardiomyocytes during ischemia before reperfusion. Am J Physiol 1999;277:H2240-6.
- Xia Y, Tsai AL, Berka V, Zweier JL. Superoxide generation from endothelial nitric-oxide synthase. J Biol Chem 1998;273:25804-8.
- Dumitrescu C, Biondi R, Xia Y, et al. Myocardial ischemia results in tetrahydrobiopterin (BH4) oxidation with impaired endothelial function ameliorated by BH4. Proc Natl Acad Sci 2007;104:15081-6.
- Tanaka M, Fujiwara H, Yamasaki K, Sasayama S. Superoxide dismutase and n-2-mercaptopropionyl glycine attenuate infarct size limitation effect of ischaemic preconditioning in the rabbit. Cardiovasc Res 1994;28:980-6.
- Pain T, Yang X-M, Critz SD, et al. Opening of mitochondrial KATP channels triggers the preconditioned state by generating free radicals. Circ Res 2000;87:460-6.
- Ferdinandy P, Schulz R. Nitric oxide, superoxide, and peroxynitrite in myocardial ischaemia-reperfusion injury and preconditioning. Br J Pharmacol 2003;138:532-43.
- Harrison DG. Perspective series: Nitric oxide and nitric oxide synthases. Cellular and molecular mechanisms of endothelial cell dysfunction. J Clin Invest 1997;100:2153-7.
- Peterson TE, Poppa V, Ueba H, Wu A, Yan C, Berk BC. Opposing effects of reactive oxygen species and cholesterol on endothelial nitric oxide synthase and endothelial cell caveolae. Circ Res 1999;85:29-37.
- Guzik TJ, Mussa S, Gastaldi D, et al. Mechanisms of increased vascular superoxide production in human diabetes mellitus: Role of NAD(P)H oxidase and endothelial nitric oxide synthase. Circulation 2002;105:1656-62.
- Chen CA, Druhan LJ, Varadharaj S, Chen YR, Zweier JL. Phosphorylation of endothelial nitric oxide synthase regulates superoxide generation from the enzyme. J Biol Chem 2008;283:27038-47.
- Stuehr DJ, Santolini J, Wang Z-Q, Wei C-C, Adak S. Update on mechanism and catalytic regulation in the NO synthases. J Biol Chem 2004;279:36167-70.
- Chatterjee A, Catravas JD. Endothelial nitric oxide (NO) and its pathologic regulation. Vascul Pharmacol 2008;49:134-40.
- Yaqoob M, Edelstein CL, Schrier RW. Role of nitric oxide and superoxide balance in hypoxia-reoxygenation proximal tubular injury. Nephrol Dial Transplant 1996;11:1743-6.
- Rengasamy A, Johns RA. Regulation of nitric oxide synthase by nitric oxide. Mol Pharmacol 1993;44:124-8.
- Kotsonis P, Frey A, Fröhlich LG, et al. Autoinhibition of neuronal nitric oxide synthase: Distinct effects of reactive nitrogen and oxygen species on enzyme activity. Biochem J 1999;340:745-52.
- Kotsonis P, Fröhlich LG, Shutenko ZV, Horejsi R, Pfleiderer W, Schmidt HH. Allosteric regulation of neuronal nitric oxide synthase by tetrahydrobiopterin and suppression of auto-damaging superoxide. Biochem J 2000;346:767-76.
- Muscari C, Bonafe F, Gamberini C, et al. Early preconditioning prevents the loss of endothelial nitric oxide synthase and enhances its activity in the ischemic/reperfused rat heart. Life Sci 2004;74:1127-37.
- Mount PF, Kemp BE, Power DA. Regulation of endothelial and myocardial NO synthesis by multi-site eNOS phosphorylation. J Mol Cell Cardiol 2007;42:271-9.
- Yang C, Talukder MA, Varadharaj S, Velayutham M, Zweier JL. Early ischaemic preconditioning requires Akt- and PKA-mediated activation of eNOS via serine 1176 phosporylation. Cardiovasc Res 2013;97:33-43.
- Gratton JP, Fontana J, O’Connor DS, Garcia-Gardena G, McCabe TJ, Sessa WC. Reconstitution of an endothelial nitric-oxide synthase (eNOS), hsp90, and caveolin-1 complex in vitro. Evidence that hsp90 facilitates calmodulin stimulated displacement of eNOS from caveolin-1. J Biol Chem 2000;275:22268-72.
- Cosentino F, Lüscher TF. Tetrahydrobiopterin and endothelial nitric oxide synthase activity. Cardiovasc Res 1999;43:274-8.
- Moens AL, Kass DA. Tetrahydrobiopterin and cardiovascular disease. Arterioscler Thromb Vasc Biol 2006;26:2439-44.
- Vasquez-Vivar J, Kalyanaraman B, Martasek P, et al. Superoxide generation by endothelial nitric oxide synthase: The influence of cofactors. Proc Natl Acad Sci USA 1998;95:9220-5.
- Heitzer T, Brockhoff C, Mayer B, et al. Tetrahydrobiopterin improves endothelium-dependent vasodilatation in chronic smokers: Evidence for a dysfunctional nitric oxide synthase. Circ Res 2000;86:E36.
- Vladic N, Ge Z-D, Leucker T, et al. Decreased tetrahydrobiopterin and disrupted association of Hsp90 with eNOS by hyperglycaemia impair myocardial ischemic preconditioning. Am J Physiol Heart Circ Physiol 2011;301:H2130-9.