The efficacy of Gonadotropin-Releasing Hormone (GNRH) agonist before frozen embryo transfer in improving pregnancy outcome and decreasing miscarriage rate in hyperandrogenic polcystic ovary syndrome women: A randomized clinical trial
Tehran
, Iran, Email: rashidi@shariati.tums.ac.ir2 Department of Biology & Embryology, Omid Fertility Center,
Tehran
, Iran, Email: saeidi@shariati.tums.ac.irTehran
, Iran, Tel: +98218490, Fax: +98218822050, Email: asamaei@razi.tums.ac.ir
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Keywords
Hyperandrogenic polycystic ovary syndrome; Frozen embryo transfer; Miscarriage rate
Introduction
Polycystic Ovarian Syndrome (PCOS) is the most common endocrine disorder among reproductive-age women Common clinical features of PCOS includes irregular menstruation, hyperandrogenism, and infertility [1]. Various diagnostic criteria have been proposed, generally centered on the features of hyperandrogenism and/or hyperandrogenemia, oligoovulation, and polycystic ovarian morphology [2]. The prevalence of PCOS is 6 – 15% in unspecified populations which depends on the studied ethnicity and criteria [3-5].
The Rotterdam 2003 criteria were developed in response to a need for broader diagnostic criteri [6]. According to the Rotterdam criteria (ROT), a woman is diagnosed with PCOS if she has two of the following manifestations: hyperandrogenism (clinical or biochemical), ovulatory dysfunction (often manifested by menstrual irregularities), and/or polycystic ovary in ultrasound [7,8].
The physiopathology of PCOS is mainly characterized by hypothalamic– pituitary–ovary axis dysfunction and anovulation. PCOS typically includes androgen excess and subtle alterations (not detected by routine tests) in serum levels of gonadotropins and estrogens [9].
It seems that the most common challenge against PCOS patients of reproductive age is infertility. Over 44% of infertile patients have shown evidence of PCOS [10]. The most important cause of infertility in these patients is ovulatory dysfunction, which results from an increase in serum levels of insulin and androgens [11]. In some PCOS patients who normally ovulate, the cause of infertility can be the negative effect of androgens on endometrial receptivity. The fertility rate in these patients remains low, despite the diversity of existing treatments suggested for ovulation stimulation in PCOS patients [12]. The high level of androgen can result in poor oocyte quality and endometrial receptivity for implantation, leading to low fertilization and high miscarriage rates in PCOS patients [13].
The focus on treatment of infertile PCOS women is among the challenging topics of infertility because the chance of fertility is low in these patients [14]. Few studies have looked into ways of reducing pregnancy complications in these patients [2].
The prevalence of early pregnancy loss is higher in PCOS women compared with normal women (30 to 50% vs.10 to 15%) [15,16], that can be attributed to impaired fibrinolysis, hyperandrogenism, increase in LH concentration, obesity, endometrial dysfunction, and insulin resistance [17].
Another cause of miscarriage in PCOS patients is hyperandrogenemia or clinical hyperandrogenism [18]. Hyperandrogenemia can have a direct effect on the quality of oocytes [19,20] and make endometrial environment inappropriate for embryo implantation and ongoing of pregnancy. In two different studies, it was discovered that elevated free/total testosterone ratios and isolated elevated free and total testosterone levels were risk factors of early pregnancy loss in PCOS women [18,21].
Therefore, treatment with GNRH agonist can reduce the serum level of androgens by suppressing or inhibiting hypothalamic-pituitary–gonadal axis, resulting in enhancement of endometrial development as well as the rate of successful pregnancy in these patients before frozen embryo transfer [22,23]. The aim of the study was to investigate the effect of treatment with GnRH agonist before frozen embryo on clinical pregnancy rates and miscarriage rates in PCOS patients who are undergoing frozen-thawed ETs.
Materials and Methods
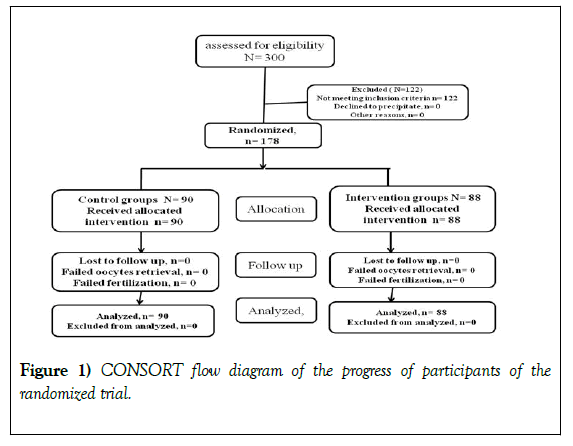
The present single blind randomized clinical trial was conducted at two infertility centers in Tehran, Iran, namely Dr Shariati Hospital affiliated with Tehran University of Medical Sciences and Omid Fertility Center, from January 2016 to October 2018. The current study was approved by the Ethics Committee of Tehran University of Medical Sciences (IR.TUMS.MEDECINE.REC.1397.096). This study was also in accordance with the principles laid down in second revision of Helsinki Declaration (1983). This investigation was also registered in Iranian Registry of Clinical Trials (IRCT20140818018842N15). The randomization was done using random number table. Written informed consent based on ethical consideration was obtained from each patient. The total number of 300 infertile PCOS patients referred to these two centers during this period. Out of these patients, 178 patients were recruited in this study (Figure 1).
Patients were randomly assigned to two groups of intervention and control. Patients and data analyzer were blinded to the study groups. Inclusion criteria were defined as follows: being infertile, being aged 18–40 years, having BMI ≤ 30 kg/m2, and being diagnosed with PCOS based on the Rotterdam criteria (suffering from at least two of three manifestations: oligo-/anovulation, hyperandrogenaemia and/or sonographic appearance of polycystic ovaries) (Rotterdam ESHRE/ASRM-sponsored PCOS consensus workshop group, 2004). Acne and hirsutism were considered as the diagnostic criteria of hyperandrogenism in study. We excluded patients who received hormonal drugs or other medications, had sever organ dysfunction or mental illness, had congenital adrenal hyperplasia or androgen–secreting neoplasm, and those with uterine abnormality, including bicornate, didelphys, or myomatous uterus.
Study Intervention
In accordance with our inclusion and exclusion criteria, patients were selected. Then, the objective of the study was explained to them. Patients were then divided into intervention and control groups by applying random number table. All infertile PCOS women in the control group received standard treatment for endometrial preparation using estradiol valerate (at dose of 6-8 mg/day) before embryo transfer. Patients in the intervention group were treated with GNRH agonist; they received two doses of GNRH agonist (Diphereline S.R. 3.75 mg, IPSEN Pharmaceutical Co. France) with an interval of 4 weeks. Two months after administrating GNRH agonist, intervention group received standard treatment for endometrial preparation using 6 mg estradiol valerate (Aburaihan Pharmaceutical Co., Tehran, Iran) daily (at dose of 6-8 mg/day). Endometrial thickness was evaluated by transvaginal ultrasonography. Progesterone intramuscular administration was started when the endometrium reached a thickness >8 mm to support the luteal phase (Fertigest, 50 mg, Aburaihan Pharmaceutical Co., Tehran, Iran). When endometrial thickness was not in the expected range, Estradiol administration was continued and increased to 8 mg per day until 3 days while the patient was examined via ultrasound. Finally, after reaching the desired endometrial thickness, maximum 2 thawed-embryos (blastocysts) were transferred under the ultrasound guidance and according to the method described earlier [24].
The luteal phase support with progesterone intramuscular injection was tapered at week 10. The primary outcome was the clinical pregnancy rate per patient and the secondary outcomes included the determination of ongoing pregnancy and miscarriage rates.
Ongoing pregnancy is defined as the continuation of pregnancy after the 12th week. Clinical pregnancy is defined as the presence of an intrauterine gestational sac with fetal cardiac activity documented by ultrasound evaluations performed 6–8 weeks after the embryo transfer procedure. A chemical pregnancy is defined as the increase in BHCG by more than 40 IU/ML [25].
Statistical Analysis
The outcome measures and associated clinical variables were analyzed using cross-tabulation analysis. Independent t-test was used to compare mean values in two groups. Chi square test was used to compare two groups using percentages. P<0.05 was considered statistically significant (SPSS V. 22.1, Microsoft, California, USA).
Results
A total number of 178 PCOS patients were included in the study, of which 88 patients were assigned to the intervention group and 90 patients to the control group. Patients in both groups were compared in terms of age, BMI, AMH, as well as FSH and LH serum levels, indicating no significant difference (Table 1).
| Variable | Control group N=90 | Intervention group N=88 | P value |
|---|---|---|---|
| Age (years) | 31.8 | 30.58 | 0.055 |
| Weight (kg) | 67.66 | 66.91 | 0.63 |
| BMI (kg/m2) | 25.981 | 25.478 | 0.384 |
| AMH (ng/ml) | 9.708 | 9.952 | 0.905 |
| Basal FSH (m IU/ml) | 5.691 | 5.948 | 0.365 |
| Basal LH (m IU/ml) | 8.616 | 7.463 | 0.688 |
| ET (endometrial thickness on first day of progesterone supplementation) (mm) | 9.203 | 9.866 | 0.014 |
| BMI: body mass index; FSH: follicle stimulating hormone; LH: luteinizing hormone. | |||
| n= the number of subjects with data. | |||
| The data were compared using Student’s t-test. | |||
Table 1:Basal characteristics of patients.
The mean age of the patients was 30.58 years old in the intervention group and 31.80 years old in the control group. With respect to mean of patients’ weight, it was 66.91 kg in the intervention group and 67.66 kg in the control group. The FSH level was 5.94 m IU/ml in the intervention group and 5.69 m IU/ml in control group. The LH level was 7.46 m IU/ml in the intervention group and 8.61 m IU/ml in control group. The endometrial thickness on the starting day of progesterone was 9.86 mm in the intervention group and 9.20 mm in the control group. The AMH was 9.95 ng/ml in the intervention group and 9.70 ng/ml in the control group. Finally, BMI was 25.47 kg/m2 in the intervention group and 25.98 kg/m2 in the control group. No significant difference was found between the two groups in terms of above mentioned variables (Table 1).
In the intervention group, 37 (42.0%) patients continued their pregnancies for more than 12 weeks and only one patient experienced miscarriage (2.6%)). Pregnancy continuation for more than 12 weeks happened in only 16 patients (18.0%) in the control group and the other 8 patients experienced miscarriage (Table 2).
| Variable | Intervention group N=88 | Control group N=90 | P value |
|---|---|---|---|
| Chemical pregnancy rate/ cycle start, n (%) | 42 (47.7%) | 32 (35.6%) | 0.099 |
| Clinical pregnancy rate/cycle start, n/n (%) | 38 (43.2%) | 24 (27.3%) | 0.027 |
| Ongoing pregnancy rate/cycle start, n/n (%) | 37(42.0%) | 16 (18.0%) | 0 |
| Early miscarriage rate/ cycle start, n/n (%) | 1 (2.6%) | 8 (33.3%) | 0.001 |
| n= the number of subjects with data. | |||
| The data were compared using Student s t-test. | |||
Table 2: Effects of GNRH agonist on implantation and pregnancy outcome
Discussion
The androgen secretion by ovarian may be gonadotropin dependent in PCOS patients. Androgen deprivation is often achieved by suppressing the release of luteinizing hormone from the anterior pituitary. Gonadotropinreleasing hormone (GnRH) is a decapeptide hormone synthesized in the hypothalamus. When GnRH is released in a pulsatile fashion, the anterior pituitary gland is stimulated to release luteinizing hormone. Luteinizing hormone induces theca cells in the ovaries, leading to androgens production. A recent study has suggested that women with polycystic ovary disease (PCOD), who have an increased follicular phase peripheral plasma LH concentration and high level circulating androgen concentrations, tend to have a poor reproductive history and an increased rate of miscarriage in particular. The rate of miscarriage in our control group (33.3%) was similar to that of other studies, suggesting that GNRH agonist can improve the result of infertility treatment in PCOS patients. Therefore, it may be possible to claim that by treating with GnRH agonists and suppressing LH and androgen that contribute to increase of miscarriage rate in PCOS patients, we can improve the outcome of pregnancy in these patients. The results of this study showed that GNRH agonist could improve implantation and pregnancy outcomes and decrease spontaneous abortion in hyperandrogenism PCOS patients.
According to findings of this study, prescription of GNRH agonist before embryo transfer led to better results in ART cycle of PCOS patients. In this clinical trial study, we demonstrated that administration of GnRH agonist before embryo transfer also led to high ongoing pregnancy rates (42.0%in the intervention group vs. 18.0% in the control group).
Pregnancy loss rates amongst women with PCOS have been reported to be as high as 30% to 50%, but some research has reported contrasting results. In a recent study, it has been revealed that women with PCOS have a 2-fold increase of miscarriage loss after undergoing ART (IVF or IUI) [26]. While the rate of miscarriage in health women is pretty substantial (11%) according to findings of a study performed by Chason et al. [26]. it is as high as 30 to 59% in women suffering from PCOS. PCOS has also been found in approximately 40% to 80% of women with recurrent miscarriages [27-29].
Studies have shown that plasma androgen concentrations are higher in patients with recurrent miscarriage and spontaneous abortions than in fertile women which can affect endometrial function [18]. Therefore, the cause of miscarriage in PCOS patients can be attributed to unfavorable condition of their endometrium. When the endometrium of a PCOS patient is compared with a fertile woman, it is seen that PCOS patients have abnormalities in endometrial development, such as an abnormal expression of HOX10 [14], Wilms tumor suppressor (WT1) [30] during the window of implantation [30], different expression of l-selectin ligands, Mucin-1, estrogen receptor, progesterone receptor (PR), and steroid metabolic enzymes [31,32].
Therefore, according to previous studies, it seems that infertility in PCOS patients after ovulation or abortion following IVF can be due to endometrial receptivity and hyperandrogenism status that have an adverse effect on the level of steroid hormones, endometrial development, and implantation. According to previous studies, in 33% of women who have normal ovulated but polycystic-appearing ovaries in the ultrasound, the androgen level is high [33]; in addition, increased testosterone and androstenedione on days 4 and 7 of luteal phase causes abortion even in non-PCOS patients [18]. Given that researchers believe that high androgen levels act as antagonize for estrogen and can adversely affect endometrial development and implantation, it seems that efforts to reduce androgens can improve pregnancy outcome in these patients [17].
One of the disadvantages of treating with GNRH agonist is that prolonged pituitary down-regulation induced by the agonist may suppress ovarian response to gonadotropin stimulation, particularly in patients with compromised ovarian reserve [34]. However, GNRH agonist treatment did not affect our study results because embryos were transmitted in freeze cycle [35]. Nevertheless, another concern is that GnRH agonist therapy may suppress response to subsequent ovarian stimulation.
GNRH agonist applies its useful role in patients' fertility in two ways: first, by reducing the amount of androgens and second, by affecting the endometrium and its developmental factors. In addition, the impact of GnRH agonist on endometrial αvβ3 vitronectin (integrin) expression has been evaluated and it is revealed that this protein may play a role in the initiation of trophoblast invasion and act as a site of interaction between the embryo and the endometrium [36,37].
It therefore seems logical that in the endometrial preparation cycle for embryo transfer in the freeze cycle using GNRH agonist, we can improve the pregnancy outcome. There are few studies in this regard [38]. In Taiwan were the first ones who studied 60 PCOS patients who were candidate for frozen embryo transfer in IVF cycle. A maximum of two doses of Leuprolide acetate was prescribed at the stage of preparing the endometrium by using estradiol valerate. In their study, clinical and ongoing pregnancy rates were significantly higher and the miscarriage rate was significantly lower in the group treated with Leuprolide acetate [38]. However, they found no significant difference between two groups in terms of clinical pregnancy rate, confirming the exclusive effect of androgen inhibition on improving endometrial development and receptivity.
Conclusion
The findings of this study suggested that treating with GNRH agonist led to better pregnancy outcomes and decrease of miscarriage rate. Accordingly, the prescription of GNRH agonist before embryo transfer in PCOS patients is recommended to improve pregnancy outcomes in this group. Moreover, the cost of treatment is low, it can be used in the freeze cycle, it does not affect the quality of follicles, and it also minimizes the probable fetal adverse effects.
Limitations
We did not study the safety of long term administration of GnRH agonist on ovarian function in PCOS patients; however, no adverse effect was reported during the course of study.
Acknowledgement
This study was performed at the Dr Shariati Hospital of Tehran University of Medical Science and Omid Fertility Center. We are thankful of all those who cooperated with us during the study.
REFERENCES
- Goodarzi MO, Dumesic DA, Chazenbalk G, et al. Polycystic ovary syndrome: etiology, pathogenesis and diagnosis. Nat Rev Endocrinol. 2011;7(4):219-31.
- Hart R. Polycystic ovarian syndrome–prognosis and treatment outcomes. Curr Opin Obstet Gynecol. 2007;19(6):529-535.
- Mehrabian F, Khani B, Kelishadi R, et al. The prevalence of polycystic ovary syndrome in Iranian women based on different diagnostic criteria. Endokrynol Pol. 2011;62(3):238-42.
- Knochenhauer E, Key T, Miller Mk, et al. Prevalence of the polycystic ovary syndrome in unselected black and white women of the southeastern United States: a prospective study. J Clin Endocrinol Metab. 1998;83(9):3078-82.
- Kauffman RP, Baker VM, DiMarino P, et al. Polycystic ovarian syndrome and insulin resistance in white and Mexican American women: a comparison of two distinct populations. Am J Obstet Gynecol. 2002;187(5):1362-9.
- Okoroh EM, Hooper WC, Atrash HK, et al. Prevalence of polycystic ovary syndrome among the privately insured,. Am J Obstet Gynecol. 2012;207(4):299.e1-7.
- Eshre TR. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81(1):19-25.
- Azziz R, Carmina E, Dewailly D, et al. Task Force on the Phenotype of the Polycystic Ovary Syndrome of The Androgen Excess and PCOS Society. Fertil Steril. 2009;91(2):456-88.
- Rocha AL, Oliveira FR, Azevedo RC, et al. Recent advances in the understanding and management of polycystic ovary syndrome. F1000Research. 2019;8.
- Gonzalez D, Thackeray H, Lewis P, et al. Loss of WT1 expression in the endometrium of infertile PCOS patients: a hyperandrogenic effect?. J Clin Endocrinol Metab. 2012;97(3):957-66.
- Homburg R, Armar N, Eshel A, et al. Influence of serum luteinising hormone concentrations on ovulation, conception, and early pregnancy loss in polycystic ovary syndrome. BMJ. 1988;297(6655):1024-6.
- Burgers JA, Fong SL, Louwers YV, et al. Oligoovulatory and anovulatory cycles in women with polycystic ovary syndrome (PCOS): what’s the difference?. J Clin Endocrinol & Metab. 2010;95(12):E485-E9.
- Pundir J, Sunkara SK, El-Toukhy T, et al. Meta-analysis of GnRH antagonist protocols: do they reduce the risk of OHSS in PCOS? Reprod Biomed Online. 2012;24(1):6-22.
- Cermik D, Selam B, Taylor HS. Regulation of HOXA-10 expression by testosterone in vitro and in the endometrium of patients with polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;88(1):238-43.
- Gray RH, Wu LY. Subfertility and risk of spontaneous abortion. Am J Public Health. 2000;90(9):1452.
- Jakubowicz DJ, Iuorno MJ, Jakubowicz S, et al. Effects of metformin on early pregnancy loss in the polycystic ovary syndrome. The J Clin Endocrinol Metab. 2002;87(2):524-9.
- Kamalanathan S, Sahoo JP, Sathyapalan T. Pregnancy in polycystic ovary syndrome. Indian J Endocrinol Metab. 2013;17(1):37.
- Okon MA, Laird SM, Tuckerman EM, et al. Serum androgen levels in women who have recurrent miscarriages and their correlation with markers of endometrial function. Fertil Steril. 1998;69(4):682-90.
- Stanger JD, Yovich JL. Reduced in vitro fertilization of human oocytes from patients with raised basal luteinizing hormone levels during the follicular phase. Br J Obstet Gynaecol. 1985;92(4):385-93.
- Howles C, Macnamee M, Edwar. Hum Reprod. 1987;2(1):17-21.
- Tulppala M, Stenman UH, Cacciatore B, et al. Polycystic ovaries and levels of gonadotrophins and androgens in recurrent miscarriage: prospective study in 50 women. Br J Obstet Gynaecol. 1993;100(4):348-52.
- Niu Z, Chen Q, Sun Y, et al. Long-term pituitary downregulation before frozen embryo transfer could improve pregnancy outcomes in women with adenomyosis. Gynecol Endocrinol. 2013;29(12):1026-30.
- Mijatovic V, Florijn E, Halim N, et al. Adenomyosis has no adverse effects on IVF/ICSI outcomes in women with endometriosis treated with long-term pituitary down-regulation before IVF/ICSI. Eur. J Obstet Gynecol Reprod Biol. 2010;151(1):62-5.
- Schoolcraft WB, Treff NR, Stevens JM, et al. Live birth outcome with trophectoderm biopsy, blastocyst vitrification, and single-nucleotide polymorphism microarray–based comprehensive chromosome screening in infertile patients. Fertil Steril. 2011;96(3):638-40.
- National Collaborating Centre for Women's and Children's Health. National Institute for Health and Clinical Excellence: Guidance. Ectopic Pregnancy and Miscarriage: Diagnosis and Initial Management in Early Pregnancy of Ectopic Pregnancy and Miscarriage. London 2012.
- Chason R, Richter K, Widra E, et al. A diagnosis of polycystic ovary syndrome (PCOS) is associated with an increased likelihood of pregnancy loss with assisted reproduction. Fertil Steril. 2010;94(4):S25.
- Palomba S, Falbo A, Orio Jr F, et al. Effect of preconceptional metformin on abortion risk in polycystic ovary syndrome: a systematic review and meta-analysis of randomized controlled trials. Fertil Steril. 2009;92(5):1646-58.
- Clifford K, Rai R, Watson H, et al. Pregnancy: An informative protocol for the investigation of recurrent miscarriage: preliminary experience of 500 consecutive cases. Hum reprod. 1994;9(7):1328-32.
- Sagle M, Bishop K, Ridley N, et al. Recurrent early miscarriage and polycystic ovaries. BMJ. 1988;297(6655):1027.
- Anthony F, Mukhtar D, Pickett M. Cameron I. Progesterone Up-Regulates WT1 mRna and Protein, and Alters the Relsative Expression of WT1 Transcripts in Cultured Endometrial Stromal Cells. J Soc Gynecol Investig. 2003;10(8):509-16.
- Margarit L, Taylor A, Roberts M, et al. MUC1 as a discriminator between endometrium from fertile and infertile patients with PCOS and endometriosis. J Clin Endocrinol Metab. 2010;95(12):5320-9.
- Margarit L, Gonzalez D, Lewis P, et al. L-selectin ligands in human endometrium: comparison of fertile and infertile subjects. Hum Reprod. 2009;24(11):2767-77.
- Decleer W, Osmanagaoglu K, Verschueren K, et al. RCT to evaluate the influence of adjuvant medical treatment of peritoneal endometriosis on the outcome of IVF. Hum Reprod. 2016;31(9):2017-23.
- Surrey ES, Minjarez DA, Schoolcraft WB. The incidence of aberrant endometrial αvβ 3 vitronectin expression in a high risk infertility population: could prolonged GnRH agonist therapy play a role?. J Assist Reprod Gen. 2007;24(11):553-6.
- Surrey ES, Minjarez DA, Schoolcraft WB. The incidence of aberrant endometrial αvβ 3 vitronectin expression in a high risk infertility population: could prolonged GnRH agonist therapy play a role? J Assist Reprod Genet. 2007;24(11):553-6.
- Creus M, Ordi J, Fábregues F, et al. The effect of different hormone therapies on integrin expression and pinopode formation in the human endometrium: a controlled study Hum Reprod. 2003;18(4):683-93.
- Lindhard A, Bentin-Ley U, Ravn V, et al. Biochemical evaluation of endometrial function at the time of implantation. Fertil Steril. 2002;78(2):221-33.
- Tsai H-W, Wang P-H, Lin L-T, et al. Using gonadotropin-releasing hormone agonist before frozen embryo transfer may improve ongoing pregnancy rates in hyperandrogenic polycystic ovary syndrome women. Gynecol Endocrinol. 2017;33(9):686-9.