THE EFFICACY OF THE AQUEOUS LEAF EXTRACT OF CORCHORUS OLITORIUS CORCHORUS OLITORIUS (JUTE) AGAINST THE DEPLETION OF THREE SELECTED MINERALS IN BLOOD OF PLASMODIUM INFECTED SWISS ALBINO MICE
2 Federal College of Veterinary and Medical Laboratory Technology, PMB 02, Vom, Plateau State, Nigeria
Received: 03-Feb-2023, Manuscript No. PULJCMID- 23-6143; Editor assigned: 06-Feb-2023, Pre QC No. PULJCMID- 23-6143(PQ); Accepted Date: Mar 15, 2023; Reviewed: 10-Feb-2023 QC No. PULJCMID- 23-6143(Q); Revised: 13-Feb-2023, Manuscript No. PULJCMID- 23-6143(R); Published: 16-Feb-2023, DOI: 10.37532/puljcmid.2023.6(1).61-68
Citation: Baiyewu O.O, Pam,V.A, Uzoigwe N.R, et al. The efficacy of the aqueous leaf extract of Corchorus olitorius (Jute) against the depletion of three selected minerals in blood of Plasmodium infected swiss albino mice. 2023; 6(1):01-08.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
BACKGROUND: Malaria is an anaemic and life-threatening disease, caused by parasites that are transmitted to people through the bites of infected female Anopheles mosquitoes, especially in the tropics. AIM: This study evaluated the efficacy of the aqueous leaf extract of Cochorus olitorus (Jute) against the depletion of three selected minerals (Calcium (Ca), Iron (Fe) and Magnesium (Mg)) in the blood of Plasmodium berghei infected Swiss Albino rats.
OBJECTIVES: To determine the phytochemical constituents of the leaf extract of Cochorus olitorus. Also, to compare the mean parasitemia as well as the estimated mineral levels in relation to the treatments of the Cochorus olitorus leaf extract. METHODOLOGY: Matured fresh leaves of C. olitorius were collected from Lafia Local Government Area of Nasarawa State. The leaves were cleaned and washed of any visible debris and cooldried in shade and then prepared by maceration. Phytochemical screening of the extracts was conducted for its qualitative and quantitative constituents. The experimental rats were administered varying curative doses (0 mg/kg, 250 mg/kg, 500 mg/kg and 1000 mg/kg) of the aqueous extract of C. olitorius once daily. Mineral supplementation and antiplasmodial activities was evaluated by a curative test, after infecting the rat models with Plasmodium berghei.
RESULTS: From the result obtained, C. olitorius indicated the presence of saponins, alkaloids, flavonoids, cardiac glycosides and anthraquinones. A high significant difference A high significant difference (P<0.001) was observed in mean parasitemia level in the Swiss Albino rats across C. olitorius treatments in which parasitemia was highest in the control group but low in the group treated with the highest dose (1000 mg/kg) of the extract.The comparison of the effect of Plasmodium infection on mean change in the 3 minerals concentrations in relation to baseline and post-baseline for each C. olitorius leaf aqueous extract treatment showed no variation (P>0.05) with the exception of two Ca groups, 500 0.05) in mean concentration between baseline and post- mg/kg and 1000 mg/kg which varied significantly (P>baseline period).
CONCLUSION: The current study has thus established the relationship between mineral supplementation and antiplasmodial activity of C. olitorius in Swiss Albino rats. The activity demonstrated by the plant was dose dependent. Hence, this study suggests the use of the C. olitorius plant for minerals supplementation and suppression of Plasmodium infection.
Key Words
Corchorus olitorius; Phytochemicals; Leaf extract; Minerals supplementation; Antiplasmodial activity; Swiss Albino Mice
Introduction
Malaria is an anaemic and life-threatening disease, caused by parasites that are transmitted to people through the bites of infected female Anopheles mosquitoes, especially in the tropics. Malaria infection causes haemolysis of infected and uninfected erythrocytes and bone marrow dyserythropoiesis which compromises rapid recovery from anaemia [1]. During intra-erythrocytic development, malaria trophozoites digest hemoglobin, which leads to parasite growth and asexual replication while accumulating toxic heme. To avoid death, the parasite synthesizes insoluble hemozoin crystals in the digestive vacuole through polymerization of -hematin dimers and in the process, the heme is converted to a high-spin ferriheme (malaria pigment) whose magnetic properties had been studied earlier [2, 3]. Iron is an essential element for blood production. About 70 percent of body's iron is found in the red blood cells of blood called hemoglobin and in muscle cells called myoglobin. Hemoglobin is essential for transferring oxygen in blood from the lungs to the tissues. Myoglobin, in muscle cells, accepts, stores, transport and releases oxygen [4]. When iron stores are exhausted, the condition is called iron depletion. Further decreases may be called iron-deficient erythropoiesis and still further decreases produce iron deficiency anemia. Corchorus olitorius is one of those green leafy vegetables rich in minerals, vitamins and antioxidants. It contains loads of iron, calcium, sodium, phosphorus, potassium, proteins, fibers, Vitamins A, C and E, riboflavin, niacin and folate. These are nutrients that help the body fight diseases and maintain good health [5]. An earlier study of the leaf extracts of Corchorus olitorius by Adegoke and Adebayo revealed that the extracts exhibit high antimicrobial activity (with respect to the standard drug used [6]. This could be adduced to the presence of the phytochemical constituents and can be of prophylactic importance. They also suggested that the highest potency demonstrated by the plant justifies its therapeutic use by traditional healers in South-Western Nigeria for gastroenteritis with good results. Calcium (Ca), Iron (Fe), Magnesium (Mg), Zinc (Zn) and Inorganic Phosphate (Pi) are essential trace elements and the plasma content of these nutrients change during the course of most infection. It was also demonstrated that some trace elements concentrations change in malaria patience. Nevertheless, it is not exactly known yet whether the causes of these changes are as a result of specific deficiencies from dietary imbalances and inadequacies or a part of defense strategies of an organism that are regulated by acute-phase proteins [7, 8]. To this end, this study investigated the efficacy of the aqueous leaf extracts of jute leave (Corchorus olitorius) against iron, calcium, and magnesium depletion in blood of Plasmodium infected Swiss Albino rats.
Materials and Methods
Plant collection
Matured fresh leaves of Corchorus olitorius were collected from Lafia Local Government Area of Nasarawa State. The authentication of the plant was done in the Department of Botany, Federal University of Lafia, Lafia, Nasarawa state, Nigeria.
Preparation of Cochorus olitorius leaf extracts
The leaves of Corchorus olitorius were cleaned and washed of any visible debris and cool-dried in shade for two weeks at room temperature. The dried leaves were crushed and pounded using mortar and pestle, and it was sieved to obtain a fine powder, using a 0.9 mm mesh-sized sieve. The plant was prepared by warm maceration [9].
Phytochemical analysis crude leaf extracts of Cochorus olitorius
The leaves of Corchorus olitorius were screened quantitatively and qualitatively for phytochemical constituents utilizing standard methods of analysis [10].
Experimental animals
Sixty-two Swiss albino rats, weighing between 20 g and 173 g were purchased from National Veterinary Research Institute Vom, Plateau State. All animals were fed with formulated feeds and water was administered ad libitum. The animals were allowed to acclimatize for a period of 3 days prior to their randomization into the various experimental groups.
Grouping and infection of swiss albino rats
Twenty four were marked and infected with Plasmodium berghei obtained from NIRPRID and randomly divided into five groups with three replicates each of 3 rats (9 rats per group). Parasites were maintained through serial blood passage in mice wherein the rat previously infected with Plasmodium berghei and with a high parasitemia served as the donor. Blood samples was taken from the donor and diluted with phosphate-buffered saline such that 0.2 ml injected intraperitoneally into the experimental ani mals contained 1×107 infected erythrocytes. The blood of the rat was screened for infection before inoculation with parasite.
Preparation of working solution
The stock solution was prepared using methods as suggested by Ibrahim, where 1 g of the each plant aqueous extract was weighed using an Electric Weighing Balance (ADAM PGW 453) [11]. The weighed extract was added to 1000 ml of distilled water and allowed to stand for 24 hours, with occasional agitation. The suspension was subsequently filtered into a volumetric flask using Whitman No. 1 filter paper and the residue washed three times with filtered portion in the volumetric flask. Final volume was adjusted to 1000 ml by adding distilled water to make the stock solution of 1000 mg/ml. from this stock solution, working solutions were prepared to obtain concentrations of 250 mg/ml, 500 mg/ml, and 1000 mg/ml. working solutions were prepared using the formula (11):
C1V1=C2V2
Where:
C1=Stock concentration (beginning concentration)
V1=Volume of stock required to prepare new solution
C2=Concentration of new or working solution (desired concentration)
V2=Volume of new solution
Treatment
The curative method described by Ryley and Peters was used with little modification. Treatment commenced following confirmation of parasitemia in the rats. Groups 2, 3 and 4 were treated with 250 mg/kg, 500 mg/kg and 1000 mg/kg respectively of the aqueous extracts of Corchorus olitorius whereas group 1 mice received 0 mg/kg/day but were not inoculated with (serving as the control). Administration of extracts was done orally once daily by gavage using a tubelin for seven days. The mineral and parasitemia level of rats was establish before every treatment was administered.
Minerals estimation
Estimation of iron concentration
The reagents (R-Acetate, R2- Ascorbic acid, R3-Ferrozine Iron calibrator) were used. The reagents were dissolved in reagent 1 buffer. It was capped and mixed gently to dissolve the content as Working Reagent (WR). 1000 µl of the working Reagent was added to the test tubes labeled as Reagent blank calibrator, sample blank and the samples. 1000 µl of reagent 3 was dropped in the reagent blank, calibrator and the sample. 200 µl of calibrator was added to the test tube calibrator and 200 µl of distilled water was added to test tube reagent blank. 200 µl of the serum sample was added to the sample blank test tubes all containing the working reagent. 200 µl of the serum sample was also added to the sample test tubes containing the working Reagents. All tubes were mixed thoroughly and incubated for 5 minutes at 37â??. The absorbance at were read at wavelength the formula below were used to calculate the concentration of Iron. Concentration of Iron=(Sample–Sample Blank–Reagent Blank)/(Standard–Reagent Blank)×100(Standard Conc.) = µg/dl (microgram/dL))
Estimation of calcium concentration
The reagents, (R-Cresophthalein complex, R2-Diethylamine and Calcium Standard) were used. The reagent was first prepared by mixing reagent (R) and reagent (R2) in the ratio 1:1 and 1000 µl was added to the test tubes labelled blank, standard and 18 samples. 10 µl of the standard reagent was added to the standard test tubes containing reagent 1. The test tubes were mixed thoroughly and allowed to incubate for 5 minutes at room temperature. BAUR SP-1104 spectrophotometer was used to measure the absorbance at wavelength of 578 nm calcium standard was used to calibrate the assay and the absorbance of standard was measured against the sample reagent blank. The formula below was used to determine the concentration of calcium.
Concentration of Calcium= (Absorbance of Sample)/(Absorban ce of Standard multiplied by concentration of standard).
Estimation of magnesium concentration
The reagents (Xylidly Blue) (110 mmol/L), Ethanolamine (PH 11.0,) (1 mol/L), GEDTA (60 mm/L) and magnesium standard were used. 1000 µl of reagents 1 (Xylidyl Blue, ethanolamine GEDTA) was added to test tubes as Blank, Standard and 18 samples. 10 µl of Magnesium standard was used as the standard of reagent. 10 µl of the serum from the centrifuge sample was taken and added to the test tube containing reagent 1. It was mixed together and allowed to incubate for 5 minutes at 37â??. BAUR SP-1104 spectrophotometer was used to measure the absorbance at wavelength of 546 nm. Small portion of the mixture were added to the curette and the absorbance of standard was measured against the reagent blank. The formula below was used to determine the magnesium concentration. Magnesium concentration=(Absorbance of sample)/ (Absorbance of standard) × Concentration of standard
Statistical analysis
Data obtained were analyzed using R Console software (Version 3.2.2). Shapiro-wilk normality test was carried out, and the data was observed to be normally distributed. One-way analysis of variance (ANOVA) was used to compare the mean parasitemia in relation to the treatments of the leaf extract. Also, ANOVA the means of estimated mineral values in relation to the treatments of the leaf extract were compared using one-way ANOVA. The level of significance was set at P<0.05.
Results
Qualitative and quantitative analysis of the phytochemicals in C. olitorius
The phytochemicals of C. olitorius leaf extract is shown in Table 1. The qualitative analysis of the phytochemicals of C. olitorius indicated the presence of saponins, alkaloids, flavonoids, cardiac glycosides and anthraquinones.
TABLE 1. Qualitative and quantitative analysis of the phytochemicals in C. olitorius
| Parameters | Qualitative Analysis | Quantitative Analysis |
|---|---|---|
| Saponins | + | 40% |
| Tannins | - | 0.52 mg/100 mg |
| Steroids | - | 3.10% |
| Alkaloids | + | 9.40% |
| flavonoids | + | 3.80% |
| Cardiac glycosides | + | Nil |
| Anthraquinone | + | Nil |
| (+)=Detected ,(-)=Undetected | ||
The quantitative analysis of the phytochemicals of C. olitorius plant revealed that the saponnins had the highest occurrence (40%), followed by alkaloids (9.4%) and flavonoids (3.8 %). The least occurrence was observed in tannins (0.52 mg/100 g)
Parasitemia in mice in relation to treatments with C. Olitorius
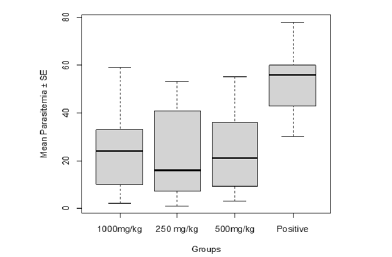
Parasitemia in relation to the treatments with C. olitorius revealed that the parasitemia was the highest in the control group, followed by 250 mg/kg and 500 mg/kg. While parasitemia was least in 1000 mg/kg treatment. Therefore, there was a very high significant difference (F80=14.76, P<0.0001, Figure 1) in the parasitemia of the mice in relation to the treatments using C. olitorius extract.
Pair-wise comparison of the mean parasitemia in mice varied significantly (P<0.0001) for positive control group versus 1000 mg/kg, positive control group versus 250 mg/kg and the positive control group versus 500 mg/kg with the exception of 250 mg/kg versus 1000 mg/kg, 500 mg/kg versus 1000 mg/kg and 250 versus 500 mg/kg which showed no significant difference as shown in Table 2.
TABLE 2. Post-hoc Tukeyâ??s multiple comparisons of means of parasitemia at 95% family-wise confidence level
| Group | Diff | Lwr | Upr | P Adj |
|---|---|---|---|---|
| 250 mg/kg-1000 mg/kg | -0.6190476 | -14.27832 | 13.04023 | 0.9993937 ns |
| 500 mg/kg-1000 mg/kg | 0.2380952 | -13.42118 | 13.89737 | 0.9999653 ns |
| Control -1000 mg/kg | 28.1428571 | 14.48358 | 41.80213 | 0.0000038* |
| 500 mg/kg-250 mg/kg | 0.8571429 | -12.80213 | 14.51642 | 0.9983987 ns |
| Control-250 mg/kg | 28.7619048 | 15.10263 | 42.42118 | 0.0000024* |
| Control- 500 mg/kg | 27.9047619 | 14.24548 | 41.56404 | 0.0000046* |
| * = Significant; ns = Not significant | ||||
Effect of plasmodium infection on the concentration of 3 minerals in the swiss albino rats before and after C. Olitorius supplementation measure
Calcium
The effect of Plasmodium infection on Calcium (Ca) concentration in relation to baseline and postbaseline concentrations for treatments with C. olitorius extract showed that there was no significant difference between the baseline and post-baseline Calcium concentration for the control group (P=0.1451) and 250 mg/kg (P=0.4314) groups as shown in Table 3. Though the comparison between baseline and post-baseline Calcium concentrations in the respective groups treated with 500 mg/kg as well as 1000 mg/kg of C. olitorius leaf extract showed a significant difference (500 mg/kg: P = 0.02137; 1000 mg/kg: P=0.002283, Table 3).
TABLE 3. The effect of Plasmodium infection on Calcium concentration in relation to baseline and post-baseline concentrations for treatments with Corchorus olitorius leaf extract
| Group | Baseline | Post-baseline | t-value | df | P value |
|---|---|---|---|---|---|
| (Mean ± SE) | (Mean ± SE) | ||||
| Control | 2.1667 ± 0.033 | 1.3500 ± 0.473 | 1.7219 | 5.0495 | 0.1451 |
| 250 mg/kg | 2.267 ± 0.088 | 1.983 ± 0.322 | 0.84641 | 5.6945 | 0.4314 |
| 500 mg/kg | 2.333 ± 0.1856 | 1.5167 ± 0.140 | 3.5126 | 4.3604 | 0.02137 |
| 1000 mg/kg | 2.467 ± 0.0333 | 1.183 ± 0.229 | 5.5541 | 5.2089 | 0.002283 |
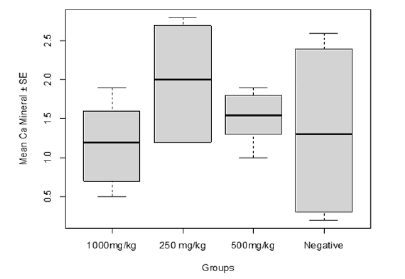
Calcium concentration in relation to post-baseline concentrations for treatments with C. olitorius leaf extract showed that the Calcium concentration was highest in the group treated with 500 mg/kg and the least was recorded in the group treated with 1000 mg/kg. Nevertheless, there was no significant difference (F20=1.188, P=0.34, Figure 2) in the Calcium concentration in relation to the treatments with C. olitorius extract.
Figure 2: Effect of Plasmodium infection on Calcium concentration in relation to post-baseline treatments with C. olitorius leaf extract
Iron (Fe)
Iron (Fe) concentration between baseline and post-baseline observation for each of the C. olitorius leaf extract treatment showed no significant variation (P > 0.05, Table 4).
TABLE 4. The effect of Plasmodium infection on Iron (Fe) concentration in relation to baseline and post-baseline concentrations for treatments with C. olitorius leaf extract
| Group | Baseline | Post-baseline | t-value | df | P value |
|---|---|---|---|---|---|
| (Mean ± SE) | (Mean ± SE) | ||||
| Control | 54.467 ± 11.085 | 55.466 ±79.489 | -0.06853 | 4.9432 | 0.948 |
| 250 mg/kg | 34.467 ± 0.960 | 35.167 ± 6.718 | -0.10315 | 5.2008 | 0.9217 |
| 500 mg/kg | 50.667 ± 19.671 | 48.817 ± 14.734 | 0.07527 | 4.3285 | 0.9434 |
| 1000 mg/kg | 52.00 ± 0.577 | 47.267 ±2.710 | 1.7082 | 5.4362 | 0.1436 |
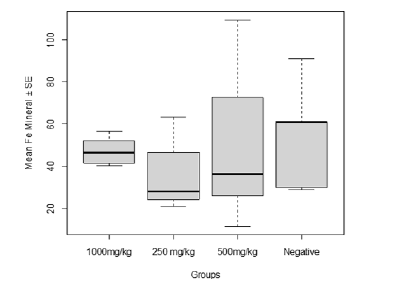
The Fe concentration in relation to post-baseline treatments with C. olitorius leaf extract despite Plasmodium infection revealed a very high Fe concentration in the control group, followed by the group treated with 500 mg/kg while the least level of concentration was observed in the group treated with 250 mg/kg. However, the concentration of Fe in relation to the various treatments with C. olitorius leaf extract showed no variation (F20 = 0.796, P = 0.511, Figure 3).
Figure 3: Effect of Plasmodium infection on the Iron (Fe) concentration in relation to post-baseline treatments with C. olitorius leaf extract
Magnesium (Mg)
Magnesium (Mg) concentration in relation to baseline and postbaseline concentration for each treatment with C. olitorius leaf extract showed no significant difference (P > 0.05) for all the groups as shown in Table 5.
TABLE 5. The effect of Plasmodium infection on Magnesium concentration in relation to baseline and post-baseline concentrations for treatments with C. olitorius leaf extract
| Group | Baseline | Post-baseline | t-value | df | P value |
|---|---|---|---|---|---|
| (Mean ± SE) | (Mean ± SE) | ||||
| Control | 1.8667 ± 0.517 | 1.3333 ±0.172 | 0.97772 | 2.4574 | 0.4144 |
| 250 mg/kg | 1.30 ± 0.0577 | 1.250 ± 0.3603 | 0.13702 | 5.2514 | 0.8961 |
| 500 mg/kg | 2.533 ± 0.484 | 0.508 ± 0.066 | 4.1435 | 2.0755 | 0.05029 |
| 1000 mg/kg | 1.767 ± 0.517 | 0.617 ± 0.070 | 2.2021 | 2.0743 | 0.154 |
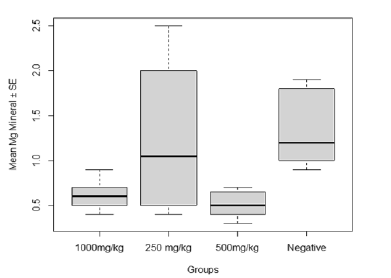
A significant difference (F20=4.269, P=0.0175, Figure 4) was observed in the post-baseline level of Mg across varying supplementation treatments with C. olitorius leaf extract due to effect of Plasmodium infection in which Mg concentration was highest in the control group, followed by the group treated with 250 mg/kg but was least in the group treated with 500 mg/kg
Figure 4: Effect of Plasmodium infection on the Magnesium (Mg) concentration in relation to post-baseline treatments with C. olitorius leaf extract
Discussion
The phytochemical constituents of C. olitorius indicated the presence of saponins, alkaloids, flavonoids, cardiac glycosides and anthraquinones. The benefits of the bioactive compounds found in the plants demonstrated a great mechanism of action beyond antioxidant activity [12, 13]. The phytochemicals observed in this study tallies with the findings of Sha’a et al. and Ikponmwosa-Eweka et al. who analyzed the phytochemical constituents of C. olitorius [14,15].
The quantitative analysis of C. olitorius plant indicated that the most abundant phytochemical was the saponnins. Saponins are known to decrease blood lipids, lower cancer risks, and lower blood glucose response. A high saponin in the blood can inhibit dental caries and platelet aggregation, in the treatment of hypercalciuria in humans, and as an antidote against acute lead poisoning [16]. This can also mean that saponins can be used to balance the mineral levels in the blood, for example; Calcium. Parasitemia in Swiss Albino rats decreased appreciably at the end of the treatment with the extracts of C. olitorius. This observation suggests that C. olitorius plant could be used to suppress the Plasmodium parasite moderately, because the parasite load in the Albino rats was almost two times lesser than those in the control group. This is congruent with an earlier findings of Karou et al. who investigated the antimalarial activity of Sida acuta (Malvaceae) and Pterocarpus erinaceus (Fabaceae) [17]. In their findings, Sida acuta belonging to the same family (Malvaceae) as C. olitorius exhibited a significant antiplasmodial activity against P. falciparum in-vitro. Another study have affirmed the efficacy of Althea officinalis L. (belonging to Malvaceae family) against P. falciparum [18]. The antiplasmodial activity observed in the current study could be as a result of the antioxidant components of the plant as antioxidant substances found naturally in the body and in plants are known to protect cell membranes from freeradical mediated oxidative damage [19]. The low parasitemia recorded in the group treated with 1000 mg/kg of C.olitorius suggests possible interaction between the plant extract and the parasite, hence the plant showed an appreciable level of antiplasmodial activity. This activity may be better enhanced when the plant is charged with other plant extracts [20]. This finding is consistent with the finding of Cudjoe et al. who evaluated selected plants for their antiplasmodial activity in vitro [21]. The effect of the infection on the Calcium concentration in relation to baseline and post-baseline concentrations showed no variation at lower concentrations of the plant extract however was improved at higher concentrations. This revealed that there was a minimal calcium level depletion activity after baseline and post-baseline examination of the albino rats. This observation may be due to the Calcium complementary role played by C. olitorius extract in the experimental organisms, as Plasmodium life cycle is strongly regulated by fluctuations in Calcium cellular levels, with deficiency causing impairment in parasite growth and invasion rate [22]. Since malaria parasites are Calcium dependent, the introduction of Calcium rich supplement without antimalarial potency may implicate the infected organism. This study agrees with the findings of Abdelsalam who observed a decrease Calcium levels on the infection with malaria [23].
The lack of variation in the iron concentration in relation to the baseline and post-baseline examinations revealed that Corchorus olitorius maintained the iron level even after infection with P. berghei, hence suggesting appreciable iron supplementation activity. This activity may be due to the high iron content in this study’s plant as suggested by Choudhary et al. who examined the nutritional profile of cultivated and wild Jute plants (Corchorus species) and recorded 67.93 mg/kg concentration of iron in the plant [24]. However, there are underlying concerns that iron deficiency may appear to offer some protection against malaria parasite, as iron supplementation may increase the vulnerability of susceptible populations to infection [25].
Magnesium concentration in relation to the treatments with Corchorus olitorius extracts revealed no variation between the baseline and post-baseline examinations, this is because the magnesium level remained the same even after infecting and treatment of the albino rats in their respective groups. The plant might have halted the rapid depletion of magnesium contents in the infected albino rats, hence the activity demonstrated. More so, haemolysis arising from the destruction of the red cells by the malaria parasites may have caused the release of magnesium from the plasma [26]. Opinions from Idirs et al. also suggest that the study plant contains high levels of magnesium [27]. This observation agrees with the findings by Oluboyo et al. who established no relationship between malaria infection and magnesium concentration [28].
Conclusion
Malaria infection causes haemolysis of both infected and uninfected blood cells and bone marrow dyserythropoiesis which compromises rapid recovery from anaemia. Malaria pathology is also known to deplete important micronutrients or minerals in the blood including, Iron, Magnesium and Calcium leading to anemia. The current study has thus established the relationship between mineral supplementaion as well as antiplasmodial activity of C. olitorius in Swiss Albino rats. The plant demonstrated an appreciable mineral supplementation activity. Hence, this study recommends the exploration of these potent plant in the treatment of mineral deficiencies due to malaria, following appropriate modification by pharmaceutical companies.
References
- White NJ. Anaemia and malaria. Malaria journal. 2018; 17(1):1-7. [Google Scholar] [Crossref]
- Moore LR, Fujioka H, Williams PS, et al. Hemoglobin degradation in malaria-infected erythrocytes determined from live cell magnetophoresis. FASEB j.: off. publ. Fed. Am. Soc. Exp. Biol. 2006;20(6):747. [Google Scholar] [Crossref]
- Pauling L, Coryell CD. The magnetic properties and structure of hemoglobin, oxyhemoglobin and carbonmonoxyhemoglobin. Proc. Natl. Acad. Sci. 1936; 22(4):210-6. [Google Scholar] [Crossref]
- Abbaspour N, Hurrell R, Kelishadi R. Review on iron and its importance for human health. J. res. med. sci.: off. j. Isfahan Univ. Med. Sci. 2014; 19(2):164. [Google Scholar]
- Famodun, O. Real Reasons Ewedu is Good for your Health.2019
- Adegoke AA, Adebayo-Tayo BC. Phytochemical composition and antimicrobial effects of Corchorous olitorius leaf extracts on four bacterial isolates. J. Med. Plants Res. 2009; 3(3):155-9. [Google Scholar] [Crossref]
- Seyrek A, Kocyigit A, Erel O. Essential trace elements selenium, zinc, copper, and iron concentrations and their related acute-phase proteins in patients with vivax malaria. Biol. trace elem. res. 2005; 106:107-15. [Google Scholar] [Crossref]
- Malaria journal
- Handa SS. An overview of extraction techniques for medicinal and aromatic plants. Extr. technol. med. aromat. plants. 2008;1(1):21-40. [Google Scholar]
- Sofowora, A. Medicinal Plants and Traditional Medicine in Africa. Spectrum Books Ltd., Ibadan, 191-289. 1993
- Ibrahim AS, Gary GK, Dennis HT. On the estimation of the most probable number in a serial dilution experiment. Commun. Stat.-Theory Methods. 1978;7(13):1267-81. [Google Scholar] [Crossref]
- Ryley JF, Peters W. The antimalarial activity of some quinolone esters. Ann. Trop. Med. Parasitol. 1970 ;64(2):
- Liu RH. Dietary bioactive compounds and their health implications. Journal of food science. 2013 ;78(s1):A18-25. [Google Scholar] [Crossref]
- Sha’a KK, Clarkson GP, Artimas SP. Phytochemical analysis, proximate composition and antinutritional factors of Corchorus oliterius plant. Int. J. Biol. Chem. Sci. 2019;13(4):2147-57. [Google Scholar] [Crossref]
- Ikponmwosa-Eweka O, Austin EI, Eluehike N, et al. Analytical comparison of the phytochemical composition and antioxdant actvity of methanol extracts derived from Alchornia cordifolia and Corchorus olitorius. J. Phytomed. Ther. 2020;19(1):364-74. [Google Scholar] [Crossref]
- Shi J, Arunasalam K, Yeung D, et al. Saponins from edible legumes: chemistry, processing, and health benefits. Journal of medicinal food. 2004 ;7(1):67-78. [Google Scholar] [Crossref]
- Karou D, Dicko MH, Sanon S, et al. Antimalarial activity of sida acuta burm. F.(Malvaceae) and pterocarpus erinaceus poir.(Fabaceae). Journal of ethnopharmacology. 2003;89(2-3):291-4. [Google Scholar] [Crossref]
- Sangian H, Faramarzi H, Yazdinezhad A, et al. Antiplasmodial activity of ethanolic extracts of some selected medicinal plants from the northwest of Iran. Parasitology research. 2013;112:3697-701. [Google Scholar] [Crossref]
- Yabani D, Adotey G. Antioxidant activity of corchorus olitorius and its effect on lipid peroxidation in mice. [Google Scholar] [Crossref]
- Arrey Tarkang P, Franzoi KD, Lee S, et al. In vitro antiplasmodial activities and synergistic combinations of differential solvent extracts of the polyherbal product, Nefang. BioMed res. Int. ;2014. [Google Scholar] [Crossref]
- Cudjoe E, Donu D, Okonu RE, et al. The in vitro antiplasmodial activities of aqueous extracts of selected Ghanaian herbal plants. J. Parasitol. Res. ;2020. [Google Scholar] [Crossref]
- de Oliveira LS, Alborghetti MR, Carneiro RG, et al. Calcium in the backstage of malaria parasite biology. Front. Cell. Infect. Microbiol. 2021;11:708834. [Google Scholar] [Crossref]
- Abdelsalam KE. Research Article Effect of Plasmodium falciparum Infection on Serum Levels of Calcium, Magnesium, Zinc and Iron Among Adult Sudanese Patients. [Google Scholar] [Crossref]
- Choudhary SB, Sharma HK, Karmakar PG, et al. Nutritional profile of cultivated and wild jute ('Corchorus') species. Australian Journal of Crop Science. 2013 Dec 1;7(13):1973-82. [Google Scholar] [Crossref]
- Spottiswoode N, Duffy PE, Drakesmith H. Iron, anemia and hepcidin in malaria. Frontiers in pharmacology. 2014 ;5:125.[ Google Scholar] [Crossref]
- Kotepui M, Piwkham D, PhunPhuech B, et al. Effects of malaria parasite density on blood cell parameters. PloS one. 2015;10(3):e0121057. [Google Scholar] [Crossref]
- IDRIS S, Yisa J, Ndamitso MM. Nutritional composition of Corchorus olitorius leaves. [Google Scholar] [Crossref]
- Oluboyo AO, Fakologbon OD, Oluboyo BO, et al. Variations in levels of selected micronutrients during malaria infection: A study from Ado-Ekiti, Ekiti, Nigeria. J. Biomed. Sci. 2018;5(1):1-6. [Google Scholar] [Crossref]