The evaluation of male infertility
- *Corresponding Author:
- Dr François Bénard
CHUM – Hôpital Saint-Luc, 301-235 boulevard Rene-Levesque, Montreal, Quebec H2X 1N8.
Telephone: 514-861-0213
Fax: 514-861-3021
E-mail simone.dekker.chum@ssss.gouv.qc.ca
Keywords
Infertility; Sperm
Significant advances have been made in our ability to evaluate and treat infertile men. This is in part due to our increased knowledge of factors associated with male subfertility, as well as recent advancements in tests and diagnostic equipment. In addition, the ability of sophisticated reproductive technology has enabled us to offer even azoospermic patients the opportunity to father a biological child. Data available over the past 25 years reveal that, in approximately one-third of cases, significant pathology is found in the man alone [1]. In 20% of cases, abnormalities are found in both the man and the woman. It is frequently recommended that clinical evaluation of the couple be delayed until one year of unprotected intercourse has passed. Because more couples wait until their late 30s to try to conceive, we believe that the initial screening of the man should be considered whenever a patient presents with the chief complaint of infertility.
Clinical Evaluation – Anotomical and Phyiological Considerations
Testicular sperm production in normal sex accessory gland function in men is possible only in an appropriate hormonal milieu. The release of gonadotropin-releasing hormone from the hypothalamus stimulates luteinizing hormone (LH) and follicle-stimulating hormone (FSH) release by the pituitary. These gonadotropins are responsible for the stimulation of testosterone production and spermatogenic function, respectively. All patients should be evaluated for possible hormonal anomalies. The average testicular volume is 20 cm3 or more in healthy young men.
Measurement of the testicular size is critical in the evaluation of infertile men because seminiferous tubules (spermatogenic regions of the testis) occupy approximately 80% of the testicular volume. A rough estimate of spermatogenic cell capacity is thus provided by assessment of testicular size. Testicular size can be estimated clinically by using an orchidometer or can be measured by using scrotal sonography. Testicular consistency is also valued in determining fertility capacity. A soft testis is likely to eject impaired spermatogenic components. Clinically, palpable induration along the course of the epididymis should raise a suspicion of epididymal obstruction. A loss of temperature differential is associated with testicular dysfunction in men with idiopathic infertility, as well as varicocele and cryptorchidism [2,3]. Over 80% of men with secondary subfertility have been found to have varicoceles that impair the couple’s fertility [1]. The right spermatic vein usually drains into the vena cava. The left spermatic vein drains into the left renal vein. The differential anatomy in the right and left spermatic veins is thought to explain, at least in part, the higher prevalence of varicoceles on the left side. The exact mechanism by which varicoceles cause infertility is unknown. In animal models, varicoceles are associated with increased blood flow to the testis and increased interstitial fluid in the testis. These two findings may impair regulation of testicular temperature and decrease intratesticular concentrations of testosterone and other local factors important for spermatogenesis. Reflux of substances from the kidney into the testicular blood flow has also been involved as a cause.
Clinical History
The history and physical examination of infertile men should be directed toward the detection of factors associated with impaired fertility. The history should initially include classification of the man’s previous fertility. Has he previously contributed to a pregnancy in his partner or a previous partner? After delineation of primary or secondary infertility for the man, the subject of sexual habits must be addressed during the initial history to evaluate the timing and frequency of intercourse. Brief assessments of the infertility evaluation of the female partner should be obtained so that an inappropriate intervention is not entertained for the man when fertility is not possible for a couple because of an unrepairable female factor.
A history of specific childhood illnesses is important in the evaluation of infertile men. A history of cryptorchidism or postpubertal mumps and orchitis, as well as the timing of pubertal landmarks and characteristics of secondary sexual development, should be noted. The development of axillary and pubic hair, the start of shaving and the density of beard (relative to male siblings) should be evaluated. Past history of bladder surgery, especially on the bladder neck, may explain a problem of retrograde ejaculation. Retroperitoneal lymph node dissection for testis tumour and other retroperitoneal surgery may disrupt sympathetic pathways necessary for emission and ejaculation. Bilateral inguinal hernia repair may cause damage to the vas deferens and may be a cause of azoospermia.
Medical conditions such as diabetes or high blood pressure may affect erection or testicular function. Diabetes can affect the sympathetic action necessary for bladder neck closure and antegrade ejaculation. Any generalized constitutional disease causing fever may temporarily impair testicular function. The effects can be detectable up to one to three months after the infection. A history of contact with chemical products such as heavy metals and pesticides should be sought. Medication, including sulfasalazine, cimetidine, nitrofurantoin and calcium channel blockers, may also impair testicular or sexual function. Any androgenic drugs such as anabolic steroids or exogenous testosterone may inhibit the function of the hypothalamic pituitary testicular axis, leading to hypogonadism. Other drugs, including alcohol, nicotine and marijuana, may adversely affect testicular function directly or indirectly [5].
Physical Examinations
The physical examination of infertile men should include a complete evaluation. Any factor that affects overall health may, theoretically, be responsible for abnormalities in sperm production. For that reason, the physical examination should be thorough, with special attention paid to the genitalia. The general appearance of men with recognized syndrome (Kallmann’s syndrome – anosmia, lack of secondary characteristics; Klinefelter’s syndrome – small testis, mental retardation) should be considered, although the diagnosis of idiopathic hypogonadotropic hypogonadism requires serum hormone evaluation. If the patient appears to be inadequately virilized, as evidenced by decreased body hair, gynecomastia, eunuchoid proportions, etc, the diagnosis of endocrine abnormality should be considered. Specific attention to the genitourinary examination is necessary. The penis should be evaluated for lesions, curvatures and Peyronie’s plaque. In hypospadiac men, the location of the urethral meatus should be noted and assessed relative to the patient’s ability to place the ejaculate deep into the vagina during intercourse. The abdomen should be palpated for abdominal masses and the inguinal regions closely inspected for scars. The scrotal examination is performed most easily in a warm room. Initially, the patient is examined in a supine position, which causes varicoceles, if present, to collapse. The consistency and size of the testis, including the presence of any testicular masses, can be evaluated in this position. Induration of the epididymis may be associated with inflammation and epididymal obstruction. If clinically indicated, a scrotal ultrasound should be performed to define the intratesticular and peritesticular structures. The presence of the vas deferens should be confirmed by palpation. Vascular engorgement of the spermatic cords should be assessed and the size of varicoceles should be recorded. Large bilateral varicoceles or a varicocele that does not decompress with the patient in a supine position may suggest an obstruction in the retroperitoneum and should be further evaluated with abdominal ultrasonography or com-puted tomography of the abdomen. A rectal examination should be performed to assess prostatic irregularity or tenderness. If clinically indicated in patients with low volume on semen analysis, a transrectal ultrasound examination should be performed to evaluate the prostate, seminal vesicles and ejaculatory ducts.
Basic Tests
Certainly, the primary source of the laboratory data is the semen analysis. Multiple analyses, at least two, spaced more than one month apart, are important to adequately evaluate fertility potential. It is important to provide patients with clearly written instructions on how to produce a specimen, and the type of container to use. Most laboratories require 48 h of abstinence. When the specimen is received in the laboratory, it is placed in an incubator at 37°C. The patient should be advised to provide a semen sample by masturbation without using any lubricant, to have the complete ejaculation placed in a jar, to bring the jar to the laboratory in less than 30 min and to keep it warm and close to his body.
The physical characteristics such as liquefaction, viscosity, volume, colour and pH are noted and recorded. A macroscopic examination is then performed. The parameters recorded are concentration, total sperm count, motility, forward progression and morphology. If no sperm are seen and if the volume is less than 1.5 cc, further tests should be done to confirm the presence of seminal vesicle fluid, as well as a postejaculate urine evaluation of the presence of spermatozoon to rule out retrograde ejaculation. If mobility is less than 50%, a viability stain coloration using eosin Y with nigrosin should be done. Greater than 50% of the sperm should be viable. Morphology is the most subjective parameter measured in the semen analysis. Table 1 shows the normal accepted parameters using World Health Organization (WHO) criteria [6].
| Sperm attribute | Normal parameter |
|---|---|
| Concentration | ≥20×109/L spermatozoa |
| Mobility | ≥25% grade A (grade 4) or |
| ≥50% grade A plus B (grade 3 or 4) | |
| Morphology | >30% normal appearing sperm |
Table 1: Normal accepted parameters of semen analysis, using the World Health Organization criteria
The normal criteria for morphology depend on which criteria are being used, either the Kruger or the WHO criteria. It is essential to ask the laboratory what is normal, depending on which criteria it uses as reference.
Hormonal Studies
An evaluation of the endocrine status of the reproductive hormonal axis (hypothalamus, pituitary and testis) is an essential component in the investigation of all male partners with either an abnormal semen examination showing less than 10×109/L spermatozoa or evidence of impaired sexual function.
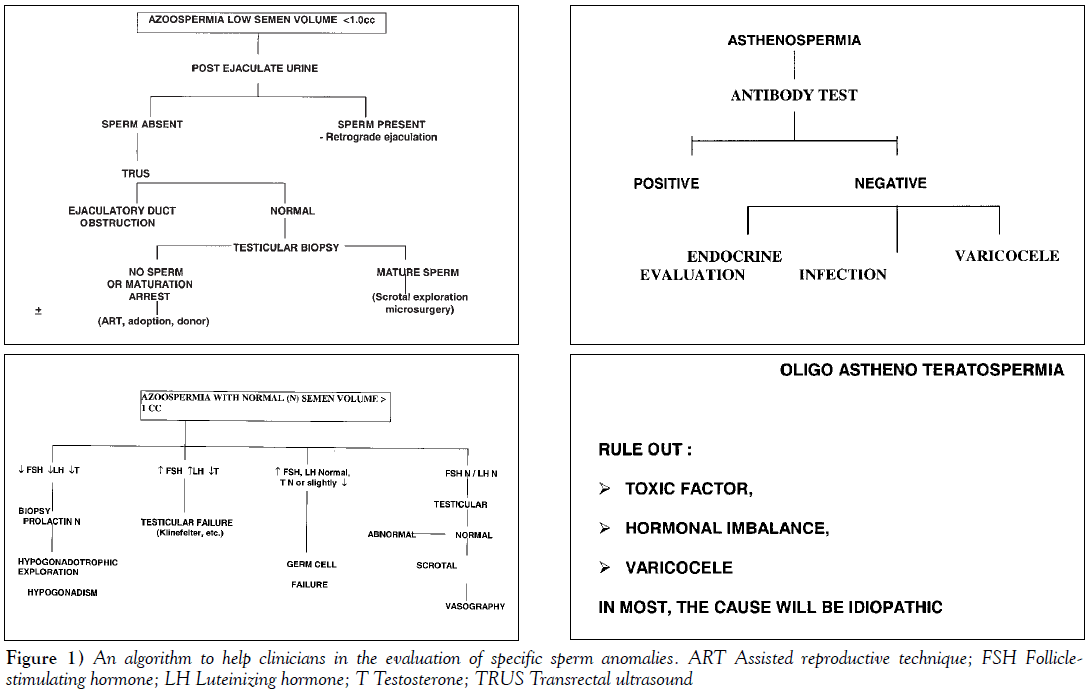
The tests to be performed depend, to a greater extent, on the clinical history and physical examination but should at least include the measurement of testosterone and FSH. If the level of testosterone is diminished, prolactin and LH levels should be measured. The significance of an abnormal hormone level is discussed in the algorithms shown in Figure 1.
Ultrasound Evaluation
An ultrasound evaluation of the genitourinary system may be very helpful. For example, the presence of utricle or ejaculatory duct cysts or seminal vesicle obstruction can be readily visualized by transrectal ultrasound. This would allow the transurethral resection of the verumontanum to be performed without the need for vasogram [7].
Scrotal ultrasound examination
Scrotal ultrasound in male factors of infertility is most helpful in the detection of epididymal or testicular mass.
Doppler colourflow studies
Doppler ultrasound is useful to identify varicoceles. A positive study is one that shows a varicocele that is larger than 2 mm in maximum diameter, provided that there is a retrograde flow during Valsalva manoeuvre. The term ‘subclinical varicocele’ has been used to describe a varicocele that is found on ultrasound examination but is undetectable by palpation. Treatment of subclinical varicocele is very controversial and most authors would agree that it is not advised at this time.
Transrectal ultrasound examination (TRUS)
Indications for TRUS in evaluating subfertile men include azoospermia with a low volume, abnormal digital rectal examination, retrograde ejaculation and suspicion of partial obstruction, meaning low volume (less than 1.5 cc).
Specialized Tests
Antisperm antibody test
Antisperm antibodies are found in approximately 10% to 15% of infertile men. Immunity to only those antigens present on the surface of viable motile sperm impair fertility. The presence of agglutination on semen analysis has often been assumed to represent the presence of sperm antibodies. Other indications for antisperm antibody testing are an abnormal postcoital test, idiopathic infertility and factors associated with increased risk of antibodies, ie, vasectomy, infection, obstruction, cryptorchidism, varicocele, testicular biopsy, testicular trauma or torsion, cancer and genetic predisposition. There are several methods to test for antisperm antibodies.
Test of sperm function
Although spermatozoa may look normal, may move with good forward progression and may be free of antibodies, this is no assurance that they perform properly. Of the many tests of sperm function available, none unequivocally identify functional spermatozoa. These tests do not need to be applied for a general evaluation for subfertile men, but they may help in the evaluation of selected patients [8].
Genetic testing
Genetic counselling for infertile patients has increased dramatically in the context of advanced reproductive technology. Before contemplating in vitro fertilization with or without intracytoplasmic sperm injection, patients should be aware of the possible transmission of disease to their children. It is well known that the risk of having abnormal karyotype or Y microdeletions in azoospermic or severely oligospermic patients is in the range of 10% to 15%. It is important to obtain karyotypes in those patients before getting involved in in vitro fertilization with the microinjection of sperm and testicular sperm extraction. In patients with congenital absence of one or both vas deferens, the risk of having a gene for cystic fibrosis of the pancreas is high (over 60%). It is important to test both sexual partners to evaluate the potential risk of having a child with cystic fibrosis. In men contemplating testicular sperm extraction for nonobstructive azoospermia, microdeletion gene evaluation is mandatory because microdeletion in certain regions of the Y chromosome may indicate that the chances of finding sperm in testicular extracts are remote.
The algorithm shown in Figure 1 may help clinicians in the evaluation of specific sperm anomalies.
Conclusion
Advances are being made rapidly in both the diagnosis and treatment of infertile men. Semen analysis is the most important test to direct the necessary evaluation. With continued work in this field and continued development of available diagnostic and treatment modalities, clinicians will be able to treat infertile couples more successfully.
References
- Goldstein M. Evaluation of the infertile male. AUA Update Series 1984;3:1-8.
- Thonneau P, Marchand S, Tallec A, et al. Incidence and main causes of infertility in a resident population (1,850,000) of three French regions (1988-1989). Hum Reprod 1991;6:811-6.
- Mieusset R, Bujan L, Mondinat C, et al. Association of scrotal hyperthermia with impaired spermatogenesis in infertile men. Fertil Steril 1987;48:1006-11.
- Gorelick J, Goldstein M. Loss of fertility in men with varicocele. Fertil Steril 1993;59:613-6.
- Schlegel P, Chang T. The testis, epididymis and ductus deferens. In: Walsh P, Retik A, Stamey T, Vaughan E, eds. Campbell’s Urology. Philadelphia: WB Saunders, 1992:190-200.
- Gilbert BR, Cooper GC, Goldstein M. Semen analysis in the evaluation of male factor subfertility. AUA Update Series 1992;11:250-5.
- Peng BC, Tomashefsky P, Nagler HM. The cofactor effect: Varicocele and infertility. Fertil Steril 1990;54:143-8.
- Liu D, Baker H. Tests of human sperm function in vitro. Fertil Steril 1992;58:465-83.
- *Corresponding Author:
- Dr François Bénard
CHUM – Hôpital Saint-Luc, 301-235 boulevard Rene-Levesque, Montreal, Quebec H2X 1N8.
Telephone: 514-861-0213
Fax: 514-861-3021
E-mail simone.dekker.chum@ssss.gouv.qc.ca
Abstract
Data available over the past 25 years regarding infertility reveal that, in approximately one-third of cases, significant pathology is found in the man alone, while in 20% of cases, abnormalities are found in both the man and the woman. It is often recommended that clinical evaluation of the couple be delayed until one year of unprotected intercourse has passed. Because more couples wait until their late 30s to try to conceive, however, the initial screening of the man should be considered whenever a patient presents with the chief complaint of infertility. Advances are being made rapidly in both the diagnosis and treatment of infertile men. Semen analysis is the most important test to direct the necessary evaluation. With continued work in this field and continued development of available diagnostic and treatment modalities, clinicians will be able to treat infertile couples more successfully
-Keywords
Infertility; Sperm
Significant advances have been made in our ability to evaluate and treat infertile men. This is in part due to our increased knowledge of factors associated with male subfertility, as well as recent advancements in tests and diagnostic equipment. In addition, the ability of sophisticated reproductive technology has enabled us to offer even azoospermic patients the opportunity to father a biological child. Data available over the past 25 years reveal that, in approximately one-third of cases, significant pathology is found in the man alone [1]. In 20% of cases, abnormalities are found in both the man and the woman. It is frequently recommended that clinical evaluation of the couple be delayed until one year of unprotected intercourse has passed. Because more couples wait until their late 30s to try to conceive, we believe that the initial screening of the man should be considered whenever a patient presents with the chief complaint of infertility.
Clinical Evaluation – Anotomical and Phyiological Considerations
Testicular sperm production in normal sex accessory gland function in men is possible only in an appropriate hormonal milieu. The release of gonadotropin-releasing hormone from the hypothalamus stimulates luteinizing hormone (LH) and follicle-stimulating hormone (FSH) release by the pituitary. These gonadotropins are responsible for the stimulation of testosterone production and spermatogenic function, respectively. All patients should be evaluated for possible hormonal anomalies. The average testicular volume is 20 cm3 or more in healthy young men.
Measurement of the testicular size is critical in the evaluation of infertile men because seminiferous tubules (spermatogenic regions of the testis) occupy approximately 80% of the testicular volume. A rough estimate of spermatogenic cell capacity is thus provided by assessment of testicular size. Testicular size can be estimated clinically by using an orchidometer or can be measured by using scrotal sonography. Testicular consistency is also valued in determining fertility capacity. A soft testis is likely to eject impaired spermatogenic components. Clinically, palpable induration along the course of the epididymis should raise a suspicion of epididymal obstruction. A loss of temperature differential is associated with testicular dysfunction in men with idiopathic infertility, as well as varicocele and cryptorchidism [2,3]. Over 80% of men with secondary subfertility have been found to have varicoceles that impair the couple’s fertility [1]. The right spermatic vein usually drains into the vena cava. The left spermatic vein drains into the left renal vein. The differential anatomy in the right and left spermatic veins is thought to explain, at least in part, the higher prevalence of varicoceles on the left side. The exact mechanism by which varicoceles cause infertility is unknown. In animal models, varicoceles are associated with increased blood flow to the testis and increased interstitial fluid in the testis. These two findings may impair regulation of testicular temperature and decrease intratesticular concentrations of testosterone and other local factors important for spermatogenesis. Reflux of substances from the kidney into the testicular blood flow has also been involved as a cause.
Clinical History
The history and physical examination of infertile men should be directed toward the detection of factors associated with impaired fertility. The history should initially include classification of the man’s previous fertility. Has he previously contributed to a pregnancy in his partner or a previous partner? After delineation of primary or secondary infertility for the man, the subject of sexual habits must be addressed during the initial history to evaluate the timing and frequency of intercourse. Brief assessments of the infertility evaluation of the female partner should be obtained so that an inappropriate intervention is not entertained for the man when fertility is not possible for a couple because of an unrepairable female factor.
A history of specific childhood illnesses is important in the evaluation of infertile men. A history of cryptorchidism or postpubertal mumps and orchitis, as well as the timing of pubertal landmarks and characteristics of secondary sexual development, should be noted. The development of axillary and pubic hair, the start of shaving and the density of beard (relative to male siblings) should be evaluated. Past history of bladder surgery, especially on the bladder neck, may explain a problem of retrograde ejaculation. Retroperitoneal lymph node dissection for testis tumour and other retroperitoneal surgery may disrupt sympathetic pathways necessary for emission and ejaculation. Bilateral inguinal hernia repair may cause damage to the vas deferens and may be a cause of azoospermia.
Medical conditions such as diabetes or high blood pressure may affect erection or testicular function. Diabetes can affect the sympathetic action necessary for bladder neck closure and antegrade ejaculation. Any generalized constitutional disease causing fever may temporarily impair testicular function. The effects can be detectable up to one to three months after the infection. A history of contact with chemical products such as heavy metals and pesticides should be sought. Medication, including sulfasalazine, cimetidine, nitrofurantoin and calcium channel blockers, may also impair testicular or sexual function. Any androgenic drugs such as anabolic steroids or exogenous testosterone may inhibit the function of the hypothalamic pituitary testicular axis, leading to hypogonadism. Other drugs, including alcohol, nicotine and marijuana, may adversely affect testicular function directly or indirectly [5].
Physical Examinations
The physical examination of infertile men should include a complete evaluation. Any factor that affects overall health may, theoretically, be responsible for abnormalities in sperm production. For that reason, the physical examination should be thorough, with special attention paid to the genitalia. The general appearance of men with recognized syndrome (Kallmann’s syndrome – anosmia, lack of secondary characteristics; Klinefelter’s syndrome – small testis, mental retardation) should be considered, although the diagnosis of idiopathic hypogonadotropic hypogonadism requires serum hormone evaluation. If the patient appears to be inadequately virilized, as evidenced by decreased body hair, gynecomastia, eunuchoid proportions, etc, the diagnosis of endocrine abnormality should be considered. Specific attention to the genitourinary examination is necessary. The penis should be evaluated for lesions, curvatures and Peyronie’s plaque. In hypospadiac men, the location of the urethral meatus should be noted and assessed relative to the patient’s ability to place the ejaculate deep into the vagina during intercourse. The abdomen should be palpated for abdominal masses and the inguinal regions closely inspected for scars. The scrotal examination is performed most easily in a warm room. Initially, the patient is examined in a supine position, which causes varicoceles, if present, to collapse. The consistency and size of the testis, including the presence of any testicular masses, can be evaluated in this position. Induration of the epididymis may be associated with inflammation and epididymal obstruction. If clinically indicated, a scrotal ultrasound should be performed to define the intratesticular and peritesticular structures. The presence of the vas deferens should be confirmed by palpation. Vascular engorgement of the spermatic cords should be assessed and the size of varicoceles should be recorded. Large bilateral varicoceles or a varicocele that does not decompress with the patient in a supine position may suggest an obstruction in the retroperitoneum and should be further evaluated with abdominal ultrasonography or com-puted tomography of the abdomen. A rectal examination should be performed to assess prostatic irregularity or tenderness. If clinically indicated in patients with low volume on semen analysis, a transrectal ultrasound examination should be performed to evaluate the prostate, seminal vesicles and ejaculatory ducts.
Basic Tests
Certainly, the primary source of the laboratory data is the semen analysis. Multiple analyses, at least two, spaced more than one month apart, are important to adequately evaluate fertility potential. It is important to provide patients with clearly written instructions on how to produce a specimen, and the type of container to use. Most laboratories require 48 h of abstinence. When the specimen is received in the laboratory, it is placed in an incubator at 37°C. The patient should be advised to provide a semen sample by masturbation without using any lubricant, to have the complete ejaculation placed in a jar, to bring the jar to the laboratory in less than 30 min and to keep it warm and close to his body.
The physical characteristics such as liquefaction, viscosity, volume, colour and pH are noted and recorded. A macroscopic examination is then performed. The parameters recorded are concentration, total sperm count, motility, forward progression and morphology. If no sperm are seen and if the volume is less than 1.5 cc, further tests should be done to confirm the presence of seminal vesicle fluid, as well as a postejaculate urine evaluation of the presence of spermatozoon to rule out retrograde ejaculation. If mobility is less than 50%, a viability stain coloration using eosin Y with nigrosin should be done. Greater than 50% of the sperm should be viable. Morphology is the most subjective parameter measured in the semen analysis. Table 1 shows the normal accepted parameters using World Health Organization (WHO) criteria [6].
| Sperm attribute | Normal parameter |
|---|---|
| Concentration | ≥20×109/L spermatozoa |
| Mobility | ≥25% grade A (grade 4) or |
| ≥50% grade A plus B (grade 3 or 4) | |
| Morphology | >30% normal appearing sperm |
Table 1: Normal accepted parameters of semen analysis, using the World Health Organization criteria
The normal criteria for morphology depend on which criteria are being used, either the Kruger or the WHO criteria. It is essential to ask the laboratory what is normal, depending on which criteria it uses as reference.
Hormonal Studies
An evaluation of the endocrine status of the reproductive hormonal axis (hypothalamus, pituitary and testis) is an essential component in the investigation of all male partners with either an abnormal semen examination showing less than 10×109/L spermatozoa or evidence of impaired sexual function.
The tests to be performed depend, to a greater extent, on the clinical history and physical examination but should at least include the measurement of testosterone and FSH. If the level of testosterone is diminished, prolactin and LH levels should be measured. The significance of an abnormal hormone level is discussed in the algorithms shown in Figure 1.
Ultrasound Evaluation
An ultrasound evaluation of the genitourinary system may be very helpful. For example, the presence of utricle or ejaculatory duct cysts or seminal vesicle obstruction can be readily visualized by transrectal ultrasound. This would allow the transurethral resection of the verumontanum to be performed without the need for vasogram [7].
Scrotal ultrasound examination
Scrotal ultrasound in male factors of infertility is most helpful in the detection of epididymal or testicular mass.
Doppler colourflow studies
Doppler ultrasound is useful to identify varicoceles. A positive study is one that shows a varicocele that is larger than 2 mm in maximum diameter, provided that there is a retrograde flow during Valsalva manoeuvre. The term ‘subclinical varicocele’ has been used to describe a varicocele that is found on ultrasound examination but is undetectable by palpation. Treatment of subclinical varicocele is very controversial and most authors would agree that it is not advised at this time.
Transrectal ultrasound examination (TRUS)
Indications for TRUS in evaluating subfertile men include azoospermia with a low volume, abnormal digital rectal examination, retrograde ejaculation and suspicion of partial obstruction, meaning low volume (less than 1.5 cc).
Specialized Tests
Antisperm antibody test
Antisperm antibodies are found in approximately 10% to 15% of infertile men. Immunity to only those antigens present on the surface of viable motile sperm impair fertility. The presence of agglutination on semen analysis has often been assumed to represent the presence of sperm antibodies. Other indications for antisperm antibody testing are an abnormal postcoital test, idiopathic infertility and factors associated with increased risk of antibodies, ie, vasectomy, infection, obstruction, cryptorchidism, varicocele, testicular biopsy, testicular trauma or torsion, cancer and genetic predisposition. There are several methods to test for antisperm antibodies.
Test of sperm function
Although spermatozoa may look normal, may move with good forward progression and may be free of antibodies, this is no assurance that they perform properly. Of the many tests of sperm function available, none unequivocally identify functional spermatozoa. These tests do not need to be applied for a general evaluation for subfertile men, but they may help in the evaluation of selected patients [8].
Genetic testing
Genetic counselling for infertile patients has increased dramatically in the context of advanced reproductive technology. Before contemplating in vitro fertilization with or without intracytoplasmic sperm injection, patients should be aware of the possible transmission of disease to their children. It is well known that the risk of having abnormal karyotype or Y microdeletions in azoospermic or severely oligospermic patients is in the range of 10% to 15%. It is important to obtain karyotypes in those patients before getting involved in in vitro fertilization with the microinjection of sperm and testicular sperm extraction. In patients with congenital absence of one or both vas deferens, the risk of having a gene for cystic fibrosis of the pancreas is high (over 60%). It is important to test both sexual partners to evaluate the potential risk of having a child with cystic fibrosis. In men contemplating testicular sperm extraction for nonobstructive azoospermia, microdeletion gene evaluation is mandatory because microdeletion in certain regions of the Y chromosome may indicate that the chances of finding sperm in testicular extracts are remote.
The algorithm shown in Figure 1 may help clinicians in the evaluation of specific sperm anomalies.
Conclusion
Advances are being made rapidly in both the diagnosis and treatment of infertile men. Semen analysis is the most important test to direct the necessary evaluation. With continued work in this field and continued development of available diagnostic and treatment modalities, clinicians will be able to treat infertile couples more successfully.
References
- Goldstein M. Evaluation of the infertile male. AUA Update Series 1984;3:1-8.
- Thonneau P, Marchand S, Tallec A, et al. Incidence and main causes of infertility in a resident population (1,850,000) of three French regions (1988-1989). Hum Reprod 1991;6:811-6.
- Mieusset R, Bujan L, Mondinat C, et al. Association of scrotal hyperthermia with impaired spermatogenesis in infertile men. Fertil Steril 1987;48:1006-11.
- Gorelick J, Goldstein M. Loss of fertility in men with varicocele. Fertil Steril 1993;59:613-6.
- Schlegel P, Chang T. The testis, epididymis and ductus deferens. In: Walsh P, Retik A, Stamey T, Vaughan E, eds. Campbell’s Urology. Philadelphia: WB Saunders, 1992:190-200.
- Gilbert BR, Cooper GC, Goldstein M. Semen analysis in the evaluation of male factor subfertility. AUA Update Series 1992;11:250-5.
- Peng BC, Tomashefsky P, Nagler HM. The cofactor effect: Varicocele and infertility. Fertil Steril 1990;54:143-8.
- Liu D, Baker H. Tests of human sperm function in vitro. Fertil Steril 1992;58:465-83.