The frequency of multidrug-resistance and extensively drug-resistant Acinetobacter baumannii in west of Iran
Received: 11-Jan-2018 Accepted Date: Feb 02, 2018; Published: 09-Feb-2018
Citation: Reza H. The frequency of multidrug-resistance and extensively drugresistant Acinetobacter baumannii in west of Iran J Clin Microbiol Infect Dis 2018;1(1):4-8.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Background and objectives: Acinetobacter baumannii is an opportunistic bacterial pathogen that can cause a variety of hospital-acquired infections, especially in the intensive care units (ICUs)and burn wards. Reportedly, one of the current globally health concerns is the prevalence of multidrug resistant and extensively drug-resistant and A. baumannii (MDR-AB, and XDR-AB). Antibiotic resistance and in-hospital survival nature of this bacterium can be attributed to its biofilm formation ability. The present study aimed to assess the antibiotic resistance pattern and the prevalence of pgaD and abaI genes in A. baumannii isolated from the clinical settings in Kermanshah, Iran.
Material and methods: Fifty isolates of A. baumannii were collected from the hospitals in Kermanshah during April 2016 to September 2017, which were identified according to API-20E system. Then, the antibiotic susceptibility test was performed for 14 antibiotics using disc diffusion method and minimum inhibitory concentration (MIC) based on microplate method for imipenem. The pgaD and abaI genes were analyzed by PCR technique.
Results: The study bacterial isolates were from burn wound samples collected from ICU and burn wards of Imam Reza and Imam Khomeini hospitals in Kermanshah. The findings showed that 50 isolates of A. baumannii were highly resistant to erythromycin, oï¬Â‚oxacin, ceftazidime, tobramycin, ticarcillin-clavulanate, ceftriaxone and azithromycin (>70%) and cefepime, piperacillin-tazobactam, ampicillin/sulbactam and amikacin (>50%) and sensitive to colistin and tigecycline. In addition, 80% of isolates were resistant to imipenem. The frequency of MDR-AB and MDR-AB strains was 84% and 48% respectively. The abaI and pga genes were found respectively in 18% and 58% of isolates.
Conclusion: High prevalence of MDR-AB isolates in Kermanshah represents the spread of resistant genes among the bacteria. The antibiotic resistance rate among A. baumannii isolates from hospitalized patients of burn and ICU wards is a warning for the region and failure to control appropriately can cause severe problems in the future.
Introduction
Acinetobacter baumannii is an aerobic, non-fermentative, gram-negative, non-motile, oxidase -negative, coccobacillus that can survive for prolonged periods in the environment and cause nosocomial infections, including urinary tract and wound infections [1,2]. This opportunistic human pathogenic bacterium develops a wide range of HAIs; for example, bacteremia, urinary tract infectious (UTI), pneumonia and secondary meningitis, but the leading harmful consequences of these bacteria emerge as HAIs especially in the ICUs and burn wards [3-5]. It is hard sto distinguish of colonization and infection from A. baumannii in the burn sites of patients. In fact, this bacterium plays a severe role in these infections as it is isolated frequently by increasing the burn patients [6,7]. On the other hand, the prevalence of A. baumannii is common in the burn wounds. In addition, the affected patients are potential reservoir for A. baumannii [8]. A. baumannii has a severe effect in the colonization and infection of hospitalized patients [9]. This bacterial prevalence in healthcare centers has increased around the world [10].
Due to the widespread use of antibiotics during the last two years, multidrug-resistance and especially extensively drug-resistant A. baumannii (MDR-AB, and XDR-AB) have accrued therefore cause a critical problem in the world [3,4]. The transmission of resistant strains among patients and the application of broad-spectrum antibiotics have been reported between wards within a hospital because of the emergence of MDR-AB [5]. The MDR-AB is associated with high mortality in the hospitalized patients [2]. In the ICU, the mortality rate associated with A. baumannii has been found to be 54% [11]. The MDR-AB in the burn wounds of the infected patients is a main risk factor for high mortality rate around the world [12]. The prevalence of these resistant strains is the leading cause of infections. Emergence of XDR-AB limits the therapeutic options and causes a serious concern to control the HAIs. For a period of 5 years (2005-2010), the frequency of XDR-AB in the clinical isolates had increased from 15% to over 41%. For the medical communities, the management of MDR-AB and XDR-AB infections is one of essential problems. It is very difficult to treat the infections caused by A. baumannii due to many multiple antibiotic resistant strains [11].
Various mechanisms of antibiotic resistance have been recognized in these microorganism, such as enzymatic degradation and modification of antibiotics, enhanced expression of efflux pump genes, reduced permeability, ability of forming the biofilm formation and altered drug targets [13], and combination therapy is usually required for effectual treatment of the nosocomial infections from A. baumannii [14]. It is ubiquitous in the hospital environment and can survive for longsome periods on dry inert surfaces [15]. The infected patients are one of the important reservoirs of A. baumannii, who might make contamination in their hands, resulting in cross transmission of the bacteria. The specified risk factors for A. baumannii colonization and infection are the protracted hospitalization, long stay in ICU, mechanical ventilation, central venous catheterization, urinary catheterization, previous exposure to antimicrobials (broad-spectrum antibiotics administration), more severity of disease, surgery, and receipt of invasive methods [16].
One of the key factors in these infectious diseases is the ability of the bacteria to form biofilm, antibiotic resistance and in-hospital survival causes of this organism have been shown to be due to its biofilm formation ability [17], due to virulence factors, such as the autoinducer synthase abaI as a part of the quorum sensing complex, and the pgaABCD operon responsible for producing poly-b-1,6-N-acetylglucosamine (PNAG) [18]. A direct relationship has been found between the biofilm formation and drug resistance in abaI [19] and pga [20] genes in the A. baumannii [21].
Objectives
Given the limited data available in the molecular characterizations of A. baumannii in Kermanshah, the current study was designed to determine the resistance pattern of different antibiotics and the presence of abaI and pgaD genes in the clinical isolates of A. baumannii in this region.
Materials and Methods
Bacterial isolates
A total of fifty clinic isolates of A. baumannii were collected during April 2016 to September 2017 from patients admitted in Imam Reza and Imam Khomeini hospitals of Kermanshah. This isolates obtained from wounds burn were identified using biochemical tests such as oxidase test, and TSI medium and Kit API 20 NE to verify the A. baumannii strains that are grown at 42°C.
Antimicrobial susceptibility test
The sensitivity of these isolates were tested using 14 antibiotics (MAST, Merseyside, UK), including ampicillin/sulbactam (10 μg/10 μg), piperacillin-tazobactam (100/10 μg), imipenem (10 μg), ceftazidime (30 μg), amikacin (30 μg), cefepime (30 μg), ceftriaxon (30 μg), ofloxacin (5 μg), tobramycin (10 μg), colistin (10 μg), azithromycin (1 μg), erythromycin (15 μg), ticarcillin-clavulanate (75 μg/10 μg) and tigecycline (15 μg) according to the CLSI table (2015) and disc diffusion method.
The resistance of bacteria strains against minimum three classes of antimicrobial agents, all penicillins and cephalosporins (including inhibitor combinations), fluroquinolones and aminoglycosides indicates the MDR-AB in this study; and resistance to the three cases of antimicrobials described above (MDR) and carbapenems refers to XDRAB [22].
The clinical laboratory standards institute (CLSI 2015) guidelines via microplate method were followed to obtain the minimum inhibitory concentration (MIC) value. The TSA-grown bacteria suspension equal to the turbidity of 0.5 McFarland standard was cultured in Muller Hinton broth (Merck, Germany) at a ratio of 1:100 to reach final concentration of 1 × 106 CFU/ml. Different dilutions of imipenem (Merck, Germany) were prepared using 0.01 M phosphate buffer. Additionally, various concentrations of 0.25-512 μg/ml from the antibiotic were provided as well. In the next step, 100 μl of different antibiotic dilutions and 100 μl of bacteria suspension were appended into each well of the microplates. The MIC value was defined as the lowest concentration inhibiting visible growth of bacteria after 24 hours incubation at 37°C.
PCR amplification
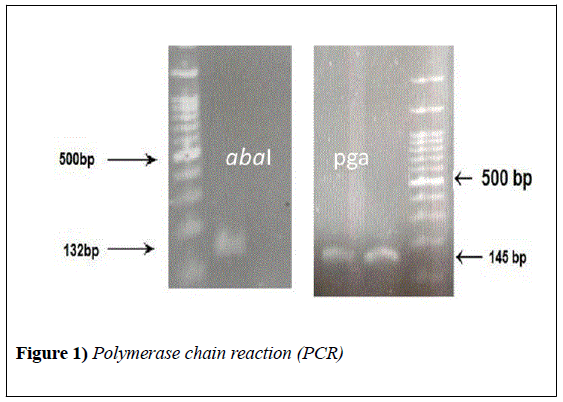
DNA template of all isolates was prepared by boiling method (30 min in 100°C). The DNA of isolates was targeted only for the abaI and pgaD genes using the primers (SinaClon, Iran) listed in Table 1.
Table 1: Primers used in this study
| Primer | Sequence (5́-3́) | Expected Amplicon Size (bp) | Reference |
|---|---|---|---|
| abaI | F: AAT GCC TAT TCC CTG CTC AC | 132 | (19) |
| R: AAT GCT TGC AGA ATT GC | |||
| pgaD | F: TTG ATC AGC CTG AAT ATG TGA | 145 | This study |
R: CAC ACA TAG TCA TAA ATG AGG
A reaction mixture (25 μl) contained 3 μl of DNA, 1 μl of each primer, 13 μl of Master Mix 2X (SinaClon, Iran), and 7 μl of deionized distilled water.
The experiment was continued according to the following program: initial denaturation at 94°C for 5 minutes, followed by 35 cycles at 94°C for 1 minute, 60°C for 1 minute, 72°C for 1 minute and final extension at 72°C for 5 minutes. The PCR products were analyzed using gel electrophoresis (1% agarose) and stained with safe dye, and visualized by Gel Doc apparatus (BioRad, USA).
Statistical analysis
Data were recorded and inserted in Excel software. Statistical analyses were performed by SPSS version 16 software using chi-square test, and Pvalue< 0.05 was considered as the statistically significance level.
Results
The bacteria were mainly isolated from burn wounds from male (62%) and female (38%) patients with the mean age of 39.4 ± 24.2 (min=1, max=73) years. The samples were collected from ICU and burn wards of Imam Reza and Imam Khomeini hospitals of Kermanshah.
A total at 50 isolates of A. baumannii were highly resistant to erythromycin, ofloxacin, ceftazidime, tobramycin, ticarcillin-clavulanate, ceftriaxone and azithromycin (>70%) and cefepime, piperacillintazobactam, ampicillin/sulbactam and amikacin (>50%) and sensitive to colistin and tigecycline (Table 2).
Table 2: Antimicrobial susceptibility testing of isolates
| Antimicrobial category | Antimicrobial agent | All isolates (n=50) | ICU (n=25) | Burn ward (n=25) | Imam Reza (n=30) | ImamKhomeini (n=20) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| I% | R% | I% | R% | I% | R% | I% | R% | I% | R % | ||
| Penicillins | Ampicilln/ sulbactam | 8 | 60 | 4 | 22 | 4 | 38 | 8 | 26 | - | 34 |
| Ticarcillin clavulanic acid | 6 | 76 | 4 | 28 | 2 | 48 | 4 | 38 | 2 | 38 | |
| Pipracilin-tazobactam | 12 | 60 | 6 | 24 | 6 | 36 | 12 | 24 | - | 36 | |
| Aminoglycosides | Amikacin | 2 | 58 | - | 14 | 2 | 44 | - | 24 | 2 | 34 |
| Tigecycline | 2 | 28 | 2 | 6 | - | 22 | 2 | 6 | - | 22 | |
| Colistin | - | 12 | - | 10 | - | 2 | - | 10 | - | 2 | |
| Tobramicin | 6 | 76 | 6 | 26 | - | 50 | - | 36 | 40 | ||
| Extended-spectrum cephalosporins; 3rd and 4th generation | Ceftazidim | - | 78 | - | 28 | - | 50 | - | 38 | - | 40 |
| Ceftriaxone | 2 | 76 | 2 | 26 | - | 50 | 2 | 36 | - | 40 | |
| Cephalosporins | Cefepime | 10 | 68 | 8 | 20 | 2 | 48 | 8 | 30 | 2 | 38 |
| Macrolides | Azithromycin | 12 | 70 | 8 | 36 | 4 | 34 | 10 | 40 | 2 | 30 |
| Erythromycin | 6 | 88 | - | 50 | 6 | 38 | 2 | 56 | 4 | 32 | |
| Carbapenem | Imipenem | 6 | 48 | 4 | 12 | 2 | 36 | 6 | 14 | - | 34 |
| Second generation Fluoroquinolones | Ofloxacin | - | 80 | - | 30 | - | 50 | - | 40 | - | 40 |
n: number, I: Intermediate, R: Resistance, -: 0%p
The MIC limits for imipenem in accordance with CLSI standard table were as follow: resistant (≥8 μg/ml), intermediate (4 μg/ml) and sensitive (≤2 μg/ml). According to the results and this protocol, imipenem-resistant isolates accounted for 80%, intermediate strains were 20% and there was no sensitive isolates.
The frequency of MDR-AB and XDR-AB was 84% and 48%, respectively.
The data were analyzed by chi-square fisher using SPSS software and Pvalue< 0.05. Accordingly, there was no relationship between the incidence of MDR-AB (p-value>0.05) and XDR-AB (P value>0.05) with abaI and pgaD genes.
The ofloxacin, cefepime, ceftazidime, ceftriaxone and azithromycin showed increasingly significant resistant in the ICU.
The abaI and pga genes were found respectively in 18% (n=9) and 58% (n=29) of isolates. As well, 6 (12%) isolates contained both abaI and pgaD genes Figure 1.
Discussion
A. baumannii is one of the nosocomial pathogens and the cause of burn wound during the last decade [5,23,24]. Unfortunately, there is a severe resistance against antimicrobial drugs like penicillins, cephalosporines, macrolides, aminoglycosides, tetracyclines and fluoroquinolones [25].
In this study, all strains of A. baumannii were isolated from the ICU and burn wards. These results are in consistent with other studies [26,27]. Our study showed that the resistance pattern of all isolates related to burn wound was similar to that reported by Tekin et al. [28].
The present study investigated the susceptibility pattern of 50 clinical strains of A. baumannii for 14 commonly used antibiotics, and the results indicated low sensitivity to the variety of antimicrobial agents in treating the infections caused by A. baumannii.
Resistance pattern of each antimicrobial agent (except for colisitin and tigecycline) was all above 50% (Table 2). The sensitivity of clinical isolates was 88% for colisitin and 70% for tigecycline. These antibiotics were found to be the most effective agents in vitro. There are only two antimicrobial agents effective in eliminating the hospital-isolated strains of A. baumannii, whereas the resistance to colisitin in A. baumannii strains is on the rise in the world [29]. In this research, the resistance to colisitin was only seen in one of the isolates in burn ward. The last lines of antibiotic therapy against A. baumannii infections are just colistin and tigecycline.
In our study, according to susceptibility test results, a considerable proportion of A. baumannii isolates (80%) were resistant to imipenem suggesting that carbapenems are unsuitable for the treatment of A. baumannii infections in burn patients hospitalized at Imam Reza and Imam Khomeini hospitals. In the present study, there was a difference between imipenem MIC results and imipenem disc diffusion. This indicated that imipenem disk could not detect 46% of resistant isolates and the use of this disc for antimicrobial susceptibility test could lead to false sensitive results.
Alavi-Moghadam et al. reported that all isolates of this bacterium were resistant to imipenem [30]. The resistant pattern of A. baumannii isolates to carbapenem investigated in this study is different to compare with other studies, probably due to geographical differences in isolates. Based on a meta-analysis in Iran, imipenem-resistant isolates of A. baumannii were 55% (95% confidence interval (CI), 53.0-56.5) and MDR isolates included 74% (95% CI, 6103-83.9) [31].
Based on a study in Iran (2006-2010), there is a similarly enhance in resistance to imipenem and meropenem of A. baumannii strains [32]. A research in a burns ward of USA showed 87% prevalence for imipenemresistant strains of A. baumannii [33].
Multidrug-resistant Acinetobacter spp. is potent pathogens often in burn ward and ICU with high outbreak, which needs epidemiologic monitoring as a measure for controlling the nosocomial infections [34].
A. baumannii strains isolated from clinical setting have shown globally increase in MDR and XDR types. The resistance of A. baumannii isolates showed that 84% were MDR, which were resistant to three groups of antibiotics including aminoglycosides, fluoroquinolones and cephalosporins. Moreover, 48% were XDR; the high prevalence of MDR and XDR causes epidemic in different geographic regions. The findings of the present study are in line with results reporting prevalent MDR A. baumannii throughout the world with 67% to 100% prevalence [35-38].
Most of the XDR isolates belonged to the patients hospitalized in burn ward. In the patients with burn wounds, the infection is the most common reason for death due to the fact that burn patients are susceptible and at high risk of infection [27]. Studies carried out in the burn and ICU wards as the main origins of HALs have isolated A. baumannii frequently [39-41].
Indiscriminate use of antimicrobial agents in the ICU, long hospital stay, prolonged use of catheters and ventilators, implants and other synthetic instruments lead to spread resistant strains colonized in susceptible patients [42].
There are serious needs to detect the key risk factors of catching HAIs, particularly ICU, to better manage and appropriate strategy to design practical protocols for infection control committees or medical doctors to adapt the best therapeutic decisions, resulting in short hospitalization, improve survival rate, reduced financial burden, low side effects, clinical decreased antibiotic resistance, and control the resistant bacteria.
In this study, the abaI and pgaD genes were seen in 18% and 58% of isolates, respectively. In a study, the genes of biofilm formation, including ompA and csuE, were detected in all samples, while bap and blaPER-1 were detected in 66% and 64% of the isolates, respectively [38]. In research of last decades, the PCR method revealed that all 30 clinical isolates of A. baumannii have the pgaD gene [20].
Conclusion
High prevalence of MDR isolates in Kermanshah region represents the spread of resistant genes among bacteria. Based on the results, an approach preventing this trend seems to be the treatment of A. baumannii infections according to the antimicrobial susceptibility testing. Our findings indicated that carbapenems are not effective against resistant isolates. Unfortunately, effective drugs such as colistin and tigecycline showed also resistance. The rate of antibiotic resistance among A. baumannii isolates from hospitalized patients of ICU and burn wards in Kermanshah is a warning for the region and failure to control appropriately can cause severe problems in the future.
REFERENCES
- Lupo A, Coyne S, Berendonk TU. Origin and evolution of antibiotic resistance: the common mechanisms of emergence and spread in water bodies. Front Microbiol. 2012;3:1-18.
- Kanafani Z, Kanj S. Acinetobacter infection: Epidemiology, microbiology, pathogenesis, clinical features, and diagnosis. Wolters Kluwer. 2013;2:21-33.
- Heslop OD, Smikle MF, Vickers IE, et al. Barton EN: High genetic diversity in human immunodeficiency virus-type 1 in Jamaica. West Indian Med J. 2009; 58:195-200.
- Bergogne-Berezin E, Towner KJ. Acinetobacter spp. as nosocomial pathogens: microbiological, clinical, and epidemiological features. Clin Microbiol Rev. 1996;9:148-65.
- Wong TH, Tan BH, Ling ML, Song C. Multi-resistant Acinetobacter baumannii on a burns unit--clinical risk factors and prognosis. Burns 2002;28:349-57.
- McConnell MJ, Actis L, Pachon J. Acinetobacter baumannii: human infections, factors contributing to pathogenesis and animal models. FEMS Microbiol Rev. 2013;37:130-55.
- Asadollahi P, Akbari M, Soroush S, et al. Antimicrobial resistance patterns and their encoding genes among Acinetobacter baumannii strains isolated from burned patients. Burns. 2012;38:1198-203.
- Barbut F, Yezli S, Mimoun M, et al. Reducing the spread of Acinetobacter baumannii and methicillin-resistant Staphylococcus aureus on a burns unit through the intervention of an infection control bundle. Burns. 2013; 39:395-403.
- Almasaudi SB. Acinetobacter spp. as nosocomial pathogens: Epidemiology and resistance features. Saudi Journal of Biological Sciences. 2016.
- Gonzalez-Villoria AM, Valverde-Garduno V. Antibiotic-Resistant Acinetobacter baumannii Increasing Success Remains a Challenge as a Nosocomial Pathogen. J Pathog. 2016.
- Deylam Salehi M, Ferdosi-Shahandashti E, Yahyapour Y, et al. Integron-Mediated Antibiotic Resistance in Acinetobacter baumannii Isolated from Intensive Care Unit Patients, Babol, North of Iran. Biomed Res Int. 2017.
- Song CT, Hwee J, Song C, et al. Burns infection profile of Singapore: prevalence of multidrug-resistant Acinetobacter baumannii and the role of blood cultures. Burns Trauma. 2016;4:1-13.
- Gurung J, Khyriem AB, Banik A, et al. Association of bio film production with multidrug resistance among clinical isolates of Acinetobacter baumannii and Pseudomonas aerugi-nosa from intensive care unit, Indian. J Crit Care Med. 2013; 17:1-214.
- Fishbain J, Peleg AY. Treatment of Acinetobacter infections. Clin Infect Dis. 2010;51:79-84.
- Alsan M, Klompas M. Acinetobacter baumannii: An Emerging and Important Pathogen. J Clin Outcomes Mana. 2010;17:363-69.
- Ellis D, Cohen B, Liu J, et al. Risk factors for hospital-acquired antimicrobial-resistant infection caused by Acinetobacter baumannii. Antimicrob Resist Infect Control. 2015;4:1-40.
- Badave GK, Kulkarni D. Biofilm Producing Multidrug Resistant Acinetobacter baumannii: An Emerging Challenge. J Clin Diagn Res. 2015.
- Longo F, Vuotto C, Donelli G. Biofilm formation in Acinetobacter baumannii. New Microbiol. 2014;37:119-27.
- He X, Lu F, Yuan F, et al. Biofilm Formation Caused by Clinical Acinetobacter baumannii Isolates Is Associated with Overexpression of the AdeFGH Efflux Pump. Antimicrob Agents Chemother. 2015;59:4817-25.
- Choi AH, Slamti L, Avci FY, et al. The pgaABCD locus of Acinetobacter baumannii encodes the production of poly-beta-1-6-N-acetylglucosamine, which is critical for biofilm formation. J Bacteriol. 2009;191:5953-63.
- Xiang J, Sun Z, Yang XG, et al. Changes in expression of gene aba I in biofilm of Acinetobacter baumannii strains isolated from burn patients. Zhonghua Shao Shang Za Zhi. 2012;28:101-5.
- Manchanda V, Sanchaita S, Singh N. Multidrug resistant acinetobacter. J Glob Infect Dis. 2010;2:291-304.
- Rennie RP, Jones RN, Mutnick AH, et al. Occurrence and antimicrobial susceptibility patterns of pathogens isolated from skin and soft tissue infections: report from the SENTRY Antimicrobial Surveillance Program (United States and Canada, 2000). Diagn Microbiol Infect Dis. 2003;45:287-93.
- AbdelRahim KA, Hassanein AM, Abd El Azeiz HA. Prevalence, plasmids and antibiotic resistance correlation of enteric bacteria in different drinking water resources in sohag, egypt. Jundishapur J Microbiol. 2015.
- Svara F, Rankin DJ. The evolution of plasmid-carried antibiotic resistance. BMC Evol Biol. 2011;11:1-130.
- Gong Y, Shen X, Huang G, et al. Epidemiology and resistance features of Acinetobacter baumannii isolates from the ward environment and patients in the burn ICU of a Chinese hospital. J Microbiol 2016;54:551-8.
- Chim H, Tan BH, Song C. Five-year review of infections in a burn intensive care unit: High incidence of Acinetobacter baumannii in a tropical climate. Burns. 2007; 33:1008-14.
- Tekin R, Dal T, Bozkurt F, et al. Risk factors for nosocomial burn wound infection caused by multidrug resistant Acinetobacter baumannii. J Burn Care Res. 2014;35:73-80.
- Cai Y, Chai D, Wang R, et al. Colistin resistance of Acinetobacter baumannii: clinical reports, mechanisms and antimicrobial strategies. J Antimicrob Chemother. 2012; 67:1607-15.
- Alavi-Moghadam M, Miri M, Mokhtari M, et al. Incidence of imipenem-resistant Acinetobacter baumannii in a general intensive care unit (ICU). Caspian J Intern Med. 2014; 5:186-7.
- Pourhajibagher M, Hashemi FB, Pourakbari B, et al. Antimicrobial Resistance of Acinetobacter baumannii to Imipenem in Iran: A Systematic Review and Meta-Analysis. Open Microbiol J. 2016;10:32-42.
- Mohajeri P, Farahani A, Feizabadi MM, et al. Antimicrobial susceptibility profiling and genomic diversity of Acinetobacter baumannii isolates: A study in western Iran. Iran J Microbiol. 2013;5:195-202.
- Trottier V, Segura PG, Namias N, et al. Outcomes of Acinetobacter baumannii infection in critically ill burned patients. J Burn Care Res. 2007; 28:248-54.
- Nimmo GR, Coombs GW, Pearson JC, et al. Methicillin-resistant Staphylococcus aureus in the Australian community: an evolving epidemic. Med J Aust. 2006; 184:384-8.
- Greene C, Vadlamudi G, Newton D, et al. The influence of biofilm formation and multidrug resistance on environmental survival of clinical and environmental isolates of Acinetobacter baumannii. Am J Infect Control. 2016;44:65-71.
- Daef EA, Mohamad IS, Ahmad AS, et al. Relationship between clinical and environmental isolates of Acinetobacter baumannii in assiut university hospitals. J Am Sci. 2013;9:67-73.
- Obeidat N, Jawdat F, Al-Bakri AG, et al. Major biologic characteristics of Acinetobacter baumannii isolates from hospital environmental and patients respiratory tract sources. Am J Infect Control. 2014;42:401-4.
- Azizi O, Shahcheraghi F, Salimizand H, et al. Molecular Analysis and Expression of bap Gene in Biofilm-Forming Multi-Drug-Resistant Acinetobacter baumannii. Rep Biochem Mol Biol. 2016;5:62-72.
- Shoja S, Moosavian M, Rostami S, et al. Dissemination of carbapenem-resistant Acinetobacter baumannii in patients with burn injuries. J Chin Med Assoc. 2017;80:245-52.
- Wroblewska MM, Towner KJ, Marchel H, et al. Emergence and spread of carbapenem-resistant strains of Acinetobacter baumannii in a tertiary-care hospital in Poland. Clin Microbiol Infect. 2007;13:490-6.
- Brusselaers N, Vogelaers D, Blot S. The rising problem of antimicrobial resistance in the intensive care unit. Ann Intensive Care. 2011;1:47.
- Harakeh S, Yassine H, Mutasem E. Antimicrobial-resistance patterns of Escherichia coli and Salmonella strains in the aquatic Lebanese environments. Environment Pollution. 2005;295:503-11.