The level of follistatin and activin in follicular fluid of long protocol in in vitro fertilization
Received: 10-Aug-2017 Accepted Date: Sep 05, 2017; Published: 12-Sep-2017
Citation: Gultekin N. The level of follistatin and activin in follicular fluid of long protocol in in vitro fertilization. J Reprod Biol Endocrinol. 2017;1(1):12-18.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
BACKGROUND: Are we estimate the success of the IVF treatment before the pregnancy test via intrafollicular hormonal markers?
OBJECTIVE: The level of follistatin and activin is measured and their levels are compared with the oocyte grade in IVF patients.
STUDY DESIGN: This study has 50 infertile patients. In our study, the best oocyte is taken from each woman. In the IVF treatment, the recombinant FSH doze around 200-300 IU. Human Chorionic Gonadotropin (HCG) 10.000 IU is administrated. The oocyte pick-up is started. The level of activin and follistatin are measured with ELISA testing. The advanced oocyte morphology grading system is used and the oocytes are divided into 4 groups.
RESULTS: In my study, it is demonstrated that the worsening of quality of oocyte by declining of level of follistatin and activin in the follicular fluid. In addition, the follistatin and activin level decrease by rising of the age.
CONCLUSION: The systemic effects of gonadotropin will be more considerable reason than the fertility in the future. For this reason, we should find the available route of the local injection of gonadotropin to infertile patients. In that point, our study has an important situation in the future.
Keywords
İnfertility; Follistatin; Activin; İntrafollicular İnjection of the Gonadotropin; İntrafollicular Hormones
The infertility means having no baby spontaneously by regular intercourse [1]. The chance of having a baby is 85%-90% in a year and the rest is infertile [1]. In 2007, 72.4 millions of World population was infertile [2]. As we know, the oocyte number declined directly by the age of women towards to menopausal age that means the most important factor was age in the infertility. The Eshre Capri Workshop mentions that the fecundity was decreased over the 30 ages [3]. The age was not only important in the ovarian reserve but also in the oocyte grade. Chuang et al. showed the pregnancy was directly related with the women age [4]. Bukman et al. searched the cornerstone of oocyte grade and ovarian reserve in infertility and they found that the age was related for both [5]. In infertility, the other factor was oocyte grade and embryo grade. The embryo grade was not only depending on the oocyte grade but it was also related with the sperm morphology. Today, many researches were done about the relation between the age and oocyte grade which showed the positive relationship and many researches showed the other effects on oocyte grade. The hormonal balance in the follicular fluid and serum that were called estradiol (E2), follicle stimulating hormone (FSH), luteinizing hormone (LH) and androgens that determined the oocyte grade. The most researches mentioned that the basal estradiol level was a parameter of induction of ovulation above 40 ages [6], the basal antral follicle number showed the canceling of IVF treatment or poor ovarian reserve [7] and the determination of poor ovarian reserve was possible with the level of basal FSH [8]. On the other hand, the effective level of all those hormones on the oocyte grade was not finding [6-8]. The oocyte maturation was a different process than the oocyte reserve and it was a developmental process as a folliculogenesis.
Our research was about the effects of follistatin and activin levels in follicular fluids on oocyte grade in long protocol of IVF treatment.
In the beginning of folliculogenesis, the growing of follicle depend on the level of FSH [9] because the FSH receptors did not found on the granulosa cells until the preantral stage [10]. In the preantral stage, the follicle had FSH receptors. In the researches, the FSH receptors found on the primer follicle and the growing of the primer follicle depends on the FSH level [11], in Kallman’s sendrom the few follicle passed through the primordial phase [12]. The follicle with the FSH receptor increased the level of FSH and LH from the pituitary with the rising of GnRH pulsatile frequency [13,14], the increasing of FSH blood level caused the aromatisation in the granulosa cells [10]. LH stimulated the theca cells to produce the androgen and the androgen was used in the synthesis of estrogen by granulosa cells [15]. The lower level of LH in granulosa cells caused the rising of aromatization instead of higher level of LH which produced more potent androgen by stimulating 5α-reductase enzyme. This androgenic environment was the reason of the follicular atresia [10]. In contrast to rising of LH level, the higher level of FSH prevented the apoptosis of follicle and it enhanced the growing of follicle [16]. Each one of ten follicles entered to preovulatuar phase and it produced more estrogen and inhibit by preventing the androgenic environment so that the follicular apoptosis stage was inhibited [17,18]. Only the dominant follicle was not affected from declining of FSH level, the dominant follicle was continued to the growing although lower FSH level [19]. The point was here, the response of dominant follicle to lower level FSH could be related to the higher amount of FSH receptor on the dominant follicle or the growth hormonal levels in intrafollicular environment [18]. In vitro research, the less effect of FSH on the cell growing by giving FSH to the granulosa cell culture or the follicle cell culture showed that the development of the dominant follicle did not only depend on FSH level but it was affected with the autocrine and the paracrine action of the intrafollicular peptides [20]. As we all know, the meaning of the dominant follicle was the having of higher amount of the estrogen concentration, higher numbers of FSH receptors and giving response to lower level of FSH. So that, the factors that caused the estorgenic environment by induction of aromatase enzyme turned the follicle to the dominant follicular phase. In most researches, the activin induced the aromatase enzyme activity [10,18]. The follistatin was an activin binding protein. For this reason, the activities of follistatin and activin on the oocyte maturation depend on their autocrine and paracrine effects in follicular fluid than their serum levels [21].
The activin was a transforming growth factor so it had the features of the transforming growth factor actions as proliferation and the differentiation on follicles [22]. The activin rose the granulosa cells and FSH receptors on the follicles and declined the induction of androgen production and increased FSH secretion from pitiutary, so that it had a positive effect on follicle development [23-25]. The intraovarian inhibin and activin application regulated the foliculogenesis and modulated the gonadotropin in immature rats [26]. The activin did not have only mitogenic activity on the granulosa cells; it had also a role on differentiation of the granulosa cells and morphogenesis of the ovarian follicle [27]. A primordial follicle had a single layered squamous cell in arrested at the prophase stage of meiotic division. In folliculogenesis, the single layer of squamous cell differentiated to the granulosa and theca cells. In in-vitro human theca cell culture, the addition of recombinant activin A inhibited the secretion of the luteinizing hormone (LH) and insulin like growth factor-1 (IGF-1) [28]. In vitro, the activin increased the aromatase enzyme activity by induction of FSH on the granulosa cells of rats [29,30]. The activin developed the ovarian follicle by decreasing of androgen production in theca cells, increasing of FSH secretion from pituitary gland and rising of insülin secretion from pancreatic β-cells [31,32]. In the polycystic ovarian syndrome (PCOS), the action of activin decreased because of binding of follistatin to activin and the androgenic environment [33].
The follistatin was proved as the activin binding protein and inhibitor of action of activin [34-36]. For this reason, the follistatin worked as an activin regulator and has a big role in the follicle development [36-38]. The follistatin had many different types [39,40], and the biochemical and physiological activity of follistatin as a 39-kDa monomerik glycoprotein [41-43]. There were very little experiments about the follistatin. In the mammarian, the follistatin was produced from two different ‘spliced messenger Ribonucleic Acid (mRNA). Those two different follistatin protein sequences were divided by exon 5 [44]. The follistatin, with 32 kDa main protein, was small follistatin and called as FS 288 [45], 35 kDa main proteins which was big follistatin is called FS 315 [43-45]. Those different structural follistatins had different activities. In the higher follistatin level, the infertility and the arrest between the first and second follicles was observed in the animal studies [46]. In vitro animal studies, the lack of the follistatin prevented the folliculogenesis and caused the infertility [47]. In the normal menstrual cycle, the midcycle rising of the follistatin was measured in contrast to polycystic ovarian syndrome [48]. In vitro, the follistatin not only worked as an activin binding protein but also regulator of hormone secretion from the pituitary gland [49-52] and degraded the activin from the circulation [53,54].
In our experiment, we tried to search; the effects of the amount of follistatin and activin in the follicular fluid on oocyte morphology during long protocol IVF treatment. In one research, the amount of activin A did not change during pituitary down regulation and recombinant FSH (rFSH) treatment [55]. However the activin A level increased with rFSH treatment in the granulose cell culture [56]. This study showed that the activin A was secreted from the granulosa cells and had the autocrine and paracrine activity. The activin rose not only aromatization but also FSH receptors on the follicle and the follicle turned to antral stage (gonadotropin dominant stage) from preantral stage and was called dominant follicle. In some researches, the level of free activin A was higher in undifferential mouse granulosa cells than the developing granulosa cells [57,58] and the lower level of activin A in follicular phase of PCOS caused the follicular arest [59]. On one study, the activin had a critical role in the oocyte maturation and was found in higher amount in the cumulus cell culture of IVF treatment [60]. This study demonstrated that the activin stimulated the oocyte maturation. All of those studies emphasis the activin effected the oocyte maturation and follistatin had a role on oocyte maturation via inhibition of activin. The circulation level of activin and follistatin was measured and compared with the oocyte morphology but the follicular level was in a mystery all the time.
Materials
• 3.75 MHz transvajinal prop (Logic 500 machine, GE, General Electric, Istanbul, Turkiye)
• Laminar flow ( K system)
• Etuv
• Incubator (Heraus BBD 6220, Germany)
• Invert microscopy (Olympus, Japan)
• Stereo microscopy (Olympus, Japan)
• Microplate reader (ahato, china)
Method
• This was a prospective study. This study has 52 infertile patients. The basal FSH, LH estradiol (E2) levels were measured. 2 patients quitted from the study because of “coasting’’. In the IVF treatment, the recombinant FSH dose was around 200-300 IU. In the folliculometre, by the measurment of the two folicles around 16.5 mm recombinant Human Chorionic Gonadotropin (HCG) 10.000 IU was administrated. After 34 h of the HCG, oocyte pick-up was started. The all follicular fluids were stored at -20°C and the level of activin and follistatin were measured with Follistatin ELISA Kit and activin-A ELISA Assay kit.
Grading of the Oocyte Morphology
The all oocytes were classified as germinal vesicles (GV), Meiosis I (MI), Meiosis II (MII). The advanced oocyte grading system was used for MII oocytes [61].
Advaced oocyte morphology grading system
GRADE 1: The oocyte was with the clear cytoplasm or a little granulation in the cytoplasm.
GRADE 2: The oocyte was with the degenerated/fragmented polar body or wide perivitelline space.
GRADE 3: The oocyte was with the granulation in the cytoplasm or dark colored cytoplasm with the bright colored zone in the center of the oocyte.
GRADE 4: The degenerated oocyte was with the endocytic cytoplasmic vacuole, granuated cytoplasm, big refrectile body or smooth endoplasmic reticulum which gave pronuclear appearance.
Including criteria
• 25-35 years old women
• Male infertility
• BMI 25 and less than 25
• Antral folicle number more than 3
• During the IVF treatment, more than 3 follicles above the 16 mm
Excluding criteria
• More than 35 years old women
• Infertility because of endometriosis
• Women with polycystic ovarian syndrome
• Poor prognosis; less than 3 follicles at 16 mm
• The estrogen level at HCG injection was not study criteria
Results
In our study, the best oocyte was taken from each woman and 50 oocytes were gathered. The advanced oocyte morphology grading system was used and the oocytes were divided into 4 groups; group a: the oocyte morphology was grade 1, group b: the oocyte morphology was grade 2, group c: the oocyte morphology was grade 3 and group d: the oocyte morphology was grade 4. There was 18 oocytes in the group a; 12 oocytes in group b; 10 oocytes in the group c and 10 oocytes in the group d. The mean age in the group a was 27,39 ± 1,54; in group b was 28,92 ± 1,51; in group c was 30,9 ± 2,51; in group d was 33,7 ± 2,26. The women in the group a were more younger than the women in the group c and d (p=0 0,0001, Mann-Whitney U test) and the women in the group b were younger than the group d (p=0,0001, Mann-Whitney U test) (Table 1 & 2).
| Group a (n=18) | Group b (n=12) | Group c (n=10) | Group d (n=10) | |
|---|---|---|---|---|
| The mean age (years) | 27.39 ± 1.54ª | 28.92 ± 1.51b | 30.9 ± 2.51ª | 33.7 ± 2.26ª,b |
| FSH level in the 3.day of cycle(IU) | 4.88 ± 1.99 | 3.8 ± 1.58 | 4.13 ± 2.13 | 5.62 ± 1.85 |
| Estrodiaol level in the 3. day of cycle (pg/ml) | 40.78 ± 18.75 | 53.88 ± 18.94 | 38 ± 13.99 | 44.44 ± 16.4 |
| Antral folicle number (AFS) | 10.22 ± 2.62 | 10.75 ± 2.86 | 9.4 ± 2.98 | 11.3 ± 3.6 |
| Stimulation time (day) | 8.39 ±1.04c | 9.17±.19 | 10.2±1.55 | 10.5 ± 1.27c |
| Total gonadotropin dose (IU) | 1637.5 ± 265.44d | 1906.25 ± 451.78e | 2315 ± 533.36d,f | 3065 ± 363.66d,e,f |
| The mean E2 level at HCG (pg/ml) | 2316.44 ± 649.71 | 2302.25 ± 1025.64 | 2410.1 ± 155.54 | 2027.9 ± 559.44 |
| The mean infertility time (years) | 4.44 ± 0.98g | 6.92 ± 1.68g | 7.6 ± 2.12g | 9.4 ± 1.26g |
* Mann-Whitney U test, p<0.05
* Mann-whitney u test, correction with bonferroni p<0.0083 (0.05/6=0.0083)
a,b,c,d,e,g p=0,0001
f p=0,001
Table 1: The all informations about the groups
| Group a (n=18) | Group b (n=12) | Group c (n=10) | Group d (n=10) | |
|---|---|---|---|---|
| Folistatin (pg/ml) | 4902.77 ± 439.13 | 4024.1667 ± 1017 | 3853 ± 924.60 | 3278 ± 928.76 |
| Activin (ng/ml) | 32.69± 11.94 | 9.22 ± 3.15 | 5.26 ± 1.85 | 2.32 ± 0.79 |
* Mann-Whitney U test, p<0.05
Table 2: The results of follistatin and activin
In the study , the infertility duration was compared and the mean time of infertility was 4,44 ± 0,98 years in the group a; 6,92 ± 1,68 years in the group b; 7,6 ± 2,12 years in the group c; 9,4 ± 1, 26 years in the group d. The duration of infertility in the group a was shorter than the other groups statistically (p=0,0001, Mann-Whitney U test).
In our study, we compared the gonadotropin treatment time ; the mean time of the gonadotropin treatment was 8,39 ± 1,04 days in group a; 9,17 ± 1,19 days in group b; 10,2 ± 1,55 days in group c; 10,5 ± 1,27 days in group d. The mean stimulation time in the group a was less than the in group d (p=0,0001, Mann-Whitney U test).
The antral follicle numbers were compared. The mean antral follicle numbers were 10,22 ± 2,62 in group a; 10,75 ± 2,86 in group b; 9,4 ± 2,98 in group c; 11,3 ± 3,6 in group d. There was no statistical importance between groups.
The level of FSH in the 3rd day of the cycle before IVF treatment was compared. The mean FSH level in the beginning was 4,88 ± 1,99 IU/L in the group a; 3,8 ± 1,58 IU/L in the group b; 4,13 ± 2,13 IU/L in the group c; 5,62 ± 1,85 IU/L in the goup d. There was no statistical importance between groups.
The serum level of the estrogen was compared at the 3rd day of the menstrual cycle. The mean estrogen level in the beginning was 40,78 ± 18,75 ng/ml in group a; 53,88 ± 18,94 ng/ml in group b; 38 ± 13,99 ng/ml in group c; 44,44 ± 16,4 ng/ml in group d. There was no statistical importance between groups.
The serum estradiol levels at the HCG application was measured and compared between groups. The maximum level of the estrogen at the HCG injection was 2316,44 ± 649,71 ng/ml in group.
The total recombinant FSH doses were compared and the mean total recombinant FSH (rFSH ) dose was 1637,5 ± 265,44 IU in group a; 1906,25 ± 451,78 IU in group b; 2315 ± 533,36 IU in group c; 3065 ± 363,66 IU in group d. The total rFSH dose was lower in group a than group c and d (p=0.0001, Mann-Whitney U test). In the group b, the rFSH dose was lower than the group d (p=0,0001, Mann-Whitney U test ). In the group c, the rFSH dose is higher than the group d (p=0,001, Mann-Whitney U test).
At the oocyte pick up, the level of follistatin and activin were measured in the folicular fluid. The mean follistatin level of folicular fluid in the group a was 4902,77 ± 439,13 pg/ml; 4024.1667 ± 1017 pg/ml in group b; 3853 ± 924.60 pg/ml in goup c; 3278 ± 928,76 pg/ml in group d. The level of follistatin in the follicular fluid was higher in group a than the other groups. The statistical importance was found between group a and group b (P=0.033, Mann-Whitney U test); between group a and group c (p=0.012, Mann- Whitney U test); between group a and group d (p=0.0001, Mann-Whitney U test).
The activin level in the follicular fluid was measured and compared in the groups. The mean activin level in the group a is 32,69 ± 11,94 ng/ml; 9.22 ± 3.15 ng/ml in group b; 5.26 ± 1.85 ng/ml in group c; 2.32 ± 0.79 ng/ ml in group d. The mean activin measurement in group a was statistically higher than other groups. The statistically importance was found between group a and group b (p=0.0001, Mann-Whitney U test); group a and group c (p=0.0001, Mann-Whitney U test); group a and group d (p=0.0001, Mann- Whitney U test).
No statistical importance was found in the follistatin level between group a and group b (P=0.033); but there was statistically importance between group a and group c (p=0.012); and between group a and group d (p=0.0001)
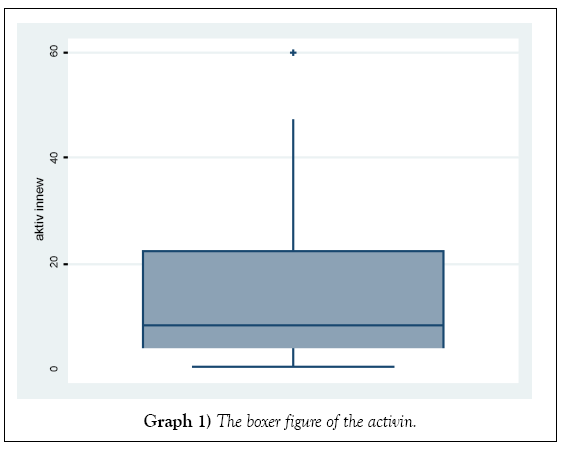
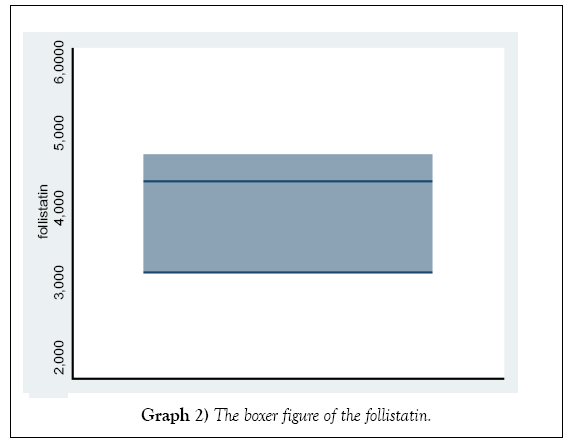
The distribution of the activin and folistatin level in Graphs 1 and 2.
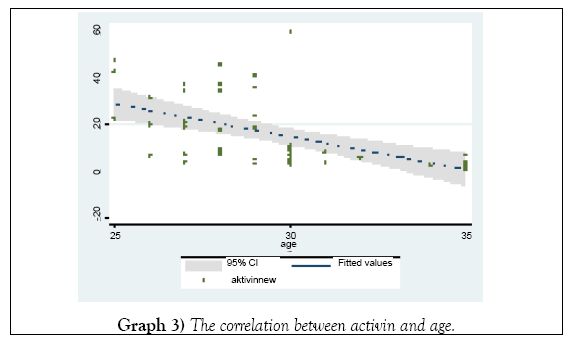
The declining in the activin levels was inversely related with the ages (Graph 3) not statistically importance
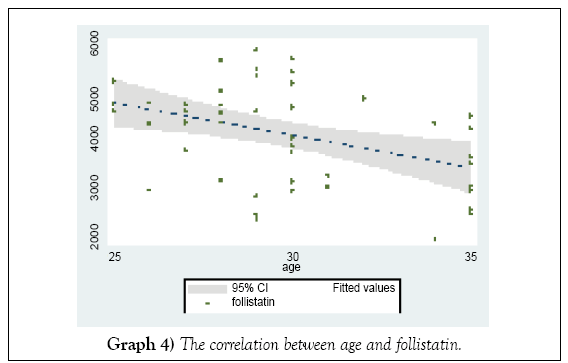
In the higher age groups, the level of the follistatin declined (Graph 4) not statistically importance
The level of the intrafolicular activin increases with the rising of the serum estrodiol level at HCG day (Graph 5).
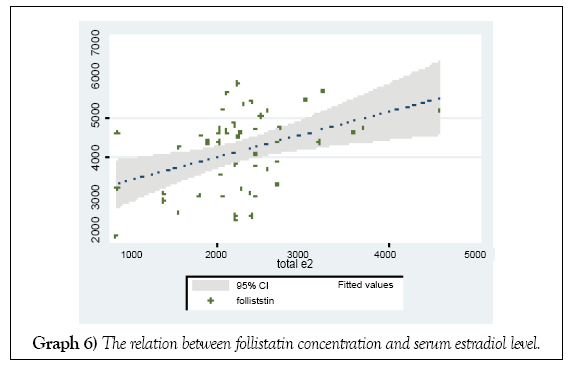
The mean intrafollicular follistatin level increased with the serum estrodiol level at HCG day (Graph 6).
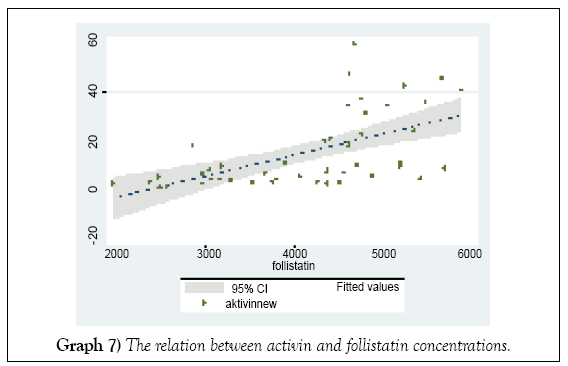
The rising of mean intrafollicular follistatin level increased the activin level at HCG day (Graph 7).
Discussion
During the foliculogenesis, the autocrine-paracrine and the endocrine factors worked in a rule for mature oocyte that had fertility capacity. The oocyte maturation depend on the granulosa cells and the follicle development depend on the oocytes [62]. The follicular growing and oocyte maturation were a complicated process that were connected to each other. The human follicular fluid analysis explained the effect of the granulosa cells on the oocyte development and maturation. In vitro studies, the nuclear maturation of the oocyte and formation of the oocyte cumulus complex were affected from activin and follistatin [63,64].
Activin had an important role on the granulosa cells for differentiation and the follicular growing. In some studies, the activin re-aggregated the granulosa cells [27,65,66] by increasing of aromatase activity which was induced by FSH, by rising of FSH receptors on the granulosa cells and by binding to activin receptors directly [67]. So that, activin induced the steriodogenesis, increased the estrogen and progesteron production, follicular development and oocyte maturation with FSH [27]. The effect of the activin on the follicle was done by binding of the activin to activin receptors on the granulosa cells oocytes [27,68]. Although the positive effect of activin on the oocyte maturation, the mechanism on oocyte morphology was not clearly demonstrated. Lau et al. found positive relation between cumulus cells, activin, oocyte morphology and embryo [60].
On the other study, the level of intrafolicular follistatin wass higher in the MΙ ve MΙΙ oocytes at ovarian stimulation [69]. For this reason, the follistatin had responsibility on oocyte quality and oocyte maturation. In the other study, the level of follistatin in the follicular fluid was higher in the MΙ ve MΙΙ oocytes [69]. The follistatin had a role on the oocyte grade and it was necessary for the oocyte maturation. In the one study, the relation between m-RNA level of the activin and follistatin level in the folicular fluid and oocyte maturation, fertilization capacity and embryo grade was searched [70]. As a result, the level of the follistatin and activin directly affected the oocyte and embryo quality. In vitro study with the mouse showed that the foliculogenesis was paused and the infertility was appeared by the lack of follistatin [47]. In our study, the follistatin was found in a higher amount at the follicular fluid of the grade 1 oocytes than the grade 2-4 oocytes but not statistically importance.
The follistatin was an activin binding protein so that it inhibited the activin [71]. The activin A level in the oocyte cumulus complex was higher in the culture of the oocyte complex medium [72]. The comparing of circulation level of the follistatin in the different age groups in the follicular phase showed the higher level of circulating follistatin among the young age groups (mean age was 23 years) than the perimenopause (mean age was 49 years) [73]. In our study, we find the decreasing level of follistatin and activin in the follicular fluid of rFSH using women by the increasing of the age.
The oocyte quality was studied in different IVF cases and the results show the basal FSH, estradiol and antral follicle did not affect the oocyte quality [74-78]. The basal estradiol in low or high level canceled the IVF cycle [74]. On the other hand, the basal FSH level showed the ovarian reserve and decreased IVF success and the cancelling of the cycle [75]. The basal antral folicle number 4 or less decreased the ovarian response to IVF treatment, caused the canceling and declining of the pregnancy rate [76]. The pregnancy rate was higher in the women less than 35 ages than above 40 ages with normal FSH/E2 level and the success of IVF was not related with the FSH level but was related with age [77]. In our study we demonstrated also the worsen of oocyte quality with the increasing of the rFSH doses and rising of age. However, we did not find any differences between basal FSH, estradiol, antral folicle number and the estradiol level in HCG injection day because those parameters were related with the oocyte reserve and they didn’t have relation with the oocyte grade [4,6,8,73]. As we seen in our study, the age was more powerful factor on the oocyte quality. We studied the follistatin and activin levels in follicular fluid with grading of oocyte quality and we demonstrated the worsening of quality of oocyte by declining of level of follistatin and activin and increasing of the age, not statistically. In other studies, the intrafollicular follistatin level in MΙ and M oocyte containing follicles was higher in IVF treatment [69] and the activin A level was higher in good quality oocyte cultures in rFSH usage [72]. In our study, the follistatin and activin levels were higher in the good quality oocytes in contrast to worse quality oocytes, not statistically. In addition, the follistatin and activin levels decreased by rising of the age. For this reason, our study figured out that the intrafollicular follistatin and activin levels declined by rising of the age and their low levels had negative effects on the oocyte grade.
Summary
This was a prospective study. We compared the level of follistatin and activin A level in the follicular fluid with the oocyte morphology in the long protocol treatment in the IVF and we determined that the follistatin and activin A level were higher in the good oocyte morphology. This study was done on limited patients because of financial reasons. For understanding the intrafollicular hormonal changes on the oocyte morphology, this study should be done on more patients. The understanding of the intrafollicular physiology in IVF treatment will provide better treatment options in poor responders and may change the treatment procedures such as applying less amount of gonadotropin directly to follicular fluid. So that the systemic effect of gonodotropin will be by-passed and successful IVF cycle can be done in minimal doses of gonadotropin even if poor responders.
Conclusion
The follistatin and activin were proteins that worked on the oocyte quality and maturation. They could be found in the circulation and in the specific parts of the human body. They seem to be counteracted each other but this study showed that they acted together in some amount and they inhibited each other in some levels during oocyte maturation. The importance of those proteins in the oocyte grade was demonstrated. Although the statistically results couldn’t be documented, the study should be reviewed or designed in a large patients group and or in the antagonist protocols.
REFERENCES
- Leon S, Marc A. Fritz: The ovary-embryology and development. Clin Gynecol Endocrinol Infertil. 2005:1013-53.
- Jacky B, Laura B, John A, et al. International estimates of infertility prevalence and treatment-seeking: Potential need and demand for infertility for medical care. Hum Reprod. 2007;22:1506-12.
- Eshre Capri Workshop Group. Fertility and ageing. Hum Reprod Update. 2005;11:261-76.
- Chuang CC, Chen CD, Chao KH, et al. Age is a better predictor of pregnancy potential than basal follicle-stimulating hormone levels in women undergoing in vitro fertilization. Fertil Steril. 2003;79:63-68.
- Bukman A, Heineman MJ. Ovarian reserve testing and the use of prognostic models in patients with subfertility. 2001;7:581-90.
- John LF, Paul AB, Michael RD, et al. Evaluation of basal estradiol levels in assisted reproductive technology cycles. Fertil Steril. 2000;74:519-24.
- John LF, Andrew JL, Bradley TM, et al. A prospective assessment of the predictive value of basal antral follicle in in vitro fertilization cycles. Fertil Steril. 2003;80:350-55.
- Buyalos RP, Daneshmand S, Brzechffa PR. Basal estradiol and follicle-stimulating hormone predict fecundity in women of advanced reproductive age undergoing ovulation induction therapy. Fertil Steril 1997;68:272-77.
- Barnes RB, Namnoum AB, Rosenfield RL, et al. The role of LH and FSH in ovarian androgen secretion and ovarian follicular development: clinical studies in a patient with isolated FSH deficiency and multicystic ovaries. Hum Reprod. 2002;17:88–91.
- Leon S, Marc A. Fritz: The ovary-embryology and development. Clin Gynecol Endocrinol Infertil. 2005:187-224 .
- Oktay K, Briggs D, Gosden RG. Ontogeny of follicle-stimulating hormone receptor gene expression in isolated human ovarian follicles. J Clin Endocrinol Metab. 1997;82:3748-51.
- Goldenberg RL, Powell RD, Rosen SW, et al. Ovarian morphology in women with anosmia and hypogonadotropic hypogonadism. Am J Obstet Gynecol. 1976;126:91-4.
- Kessel B, Dahl KD, Kazer RR, et al. The dependency of bioactive follicle-stimulating hormone secretion on gonadotropin-releasing hormone in hypogonadal and cycling women. J Clin Endocrinol Metab. 1988;66:361-66.
- Hall JE, Schoenfeld DA, Martin KA, et al. Hypothalamic gonadotropin releasing hormone secretion and follicle-stimulating hormone dynamics during the luteal-follicular transition. J Clin Endocrinol Metab. 1992;74:600–7.
- Ryan KJ, Petro Z, Kaiser J. Steroid formation by isolated and recombined ovarian granulosa and tehcal cells. J Clin Endocrinol Metab. 1968;28:355-8.
- Macklon NS, Stouffer RL, Giudice LC, et al. The science behind 25 years of ovarian stimulation for in vitro fertilization. Endocr Rev. 2006;27:170-207.
- Fauser BCJM, Vanheusden AM. Manipulation of human ovarian function: Physiological concepts and clinical consequences. Endocr Rev. 1997;18:71-106.
- McGee EA, Hsueh AJW. Initial and cyclic recruitment of ovarian follicles. Endocr Rev. 2000;21:200-14.
- Vansantbrink EJP, Vandessel HJHM, Hop WC, et al. Decremental follicle-stimulating hormone and dominant follicle development during the normal menstrual cycle. Fertil Steril. 1995;64:37-43.
- Hsueh A, Adashi E, Jones P, et al. Hormonal regulation of differentiation of cultured ovarian granulosa cells. Endocr Rev. 1984;45:76-127.
- Mather JP, Woodruff TK, Krumman L. Paracrine regulation of reproductive function by inhibin and activin. Proc Soc Exp Biol Med. 1992;201:1-15.
- Moses HL, Yang EY, Pietenpol JA. TGF-b stimulation, inhibition of cell proliferation: New mechanistic insights. Cell. 1990;63:245-7.
- Li R, Phillips DM, Mather JP. Activin promotes ovarian follicle development in vitro. Endocrinology. 1995;136:849-56.
- Silva CC, Knignht PG. Modulatory action of activin-A and follistatin on the developmental competence of in vitro-matured bovine oocytes. Biol Reprod. 1998;58:558-65.
- Smitz J, Cortvrindt R, Hu Y, et al. Effects of recombinant activin A on in vitro culture of mouse preantral follicles. Mol Reprod Dev. 1998;50:294-304.
- Woodruff TK, Lyon RJ, Hansen SE, et al. Inhibin and activin locally regulate rat ovarian folliculogenesis. Endocrinology. 1990;127:3196-205.
- Hillier SG, Yong EL, Illingworth PI, et al. Effect of recombinant activin on androgen synthesis in cultured human theca cells. J Clin Endocrinol. 1991b;72:1206-11.
- Hutchison LA, Findlay JK, De Vos FL, et al. Effect of bovine inhibin, transforming growth factor-b and bovine activin A on granulosa cell differentiation. Biochem Biophys Res Commun. 1987;146:1405-12.
- Miro F, Smyth CD, Hillier SG. Development related effects of recombinant activin on steroid synthesis in rat granulosa cells. Endocrinology. 1991;129:3388-94.
- Florlo P, Luis S, Marchett P, et al. Activin A stimulates insulin secretion in cultured human pancreatic islets. J Endocrinol Invest. 2000;23:231-4.
- Mather JP, Moore A, Li RH. Activins, inhibins and follistatins: Further thoughts on growing family of regulators. Proc Soc Exp Biol Med. 1997;215:209-22.
- Eldor T, Geva Spitz IM, Groome NP, et al. Follistatin and activin A serum concentrations in obese and non-obese patients with polycystic ovary syndrome. Hum Reprod. 2001;2552-6.
- Hillier SG, Miro F. Inhibin, activin and follistatin. Potential roles in ovarian physiology. Ann N Y Acad Sci. 1993;687:29-38.
- De Winter JP, Ten Dijke P, De Vries CJ, et al. Follistatins neutralize activin bioactivity by inhibition of activin binding to its type II receptors. Mol Cell Endocrinol. 1996;116:105-14.
- Hillier SG. Intragonadal regulation of male and female reproduction. Ann Endocrinol (Paris). 1999;60:111-7.
- Findlay JK. An update on the role of inhibin, activin and follistatin as local regulators of folliculogenesis. Biol Reprod. 1993;48:15-23.
- Schneyer AL, Hall HA, Lanbert-Messerlian G, et al. Follistatin activin complexes in human serum and follicular fluid differ immunologically and biochemically. Endocrinology. 1996;137:240-7.
- Kogava K, Nakamura T, Sugino K, et al. Activin-binding protein is present in pituitary. Endocrinology. 1991;128:1434-40.
- Nakamura T, Takio T, Eto Y, et al. Activin-binding protein from rat ovary is follistatin. Science. 1990;247:836-38.
- Mather JP, Roberts PE, Krummen LA. Follistatin modulates activin activity in a cell-and tissue-specific manner. Endocrinology. 1993;132:2732-4.
- Esh FS, Shinasaki S, Mercadao M, et al. Structural characterization of follistatin: A novel follicle stimulating hormone releasing inhibiting polypeptide from the gonad. Mol Cell Endocrinol. 1987;1:849-55.
- Inouye S, Guo Y, Depaolo L, et al. Recombinant expression of human follistatin with 315 & 288 amino acid: Chemical & biological comparison with native porcine follistatin. Endocrinology. 1991;129:815-22.
- Shimasaki S, Koga M, Esh F, et al. Primary structure of the human follistatin precursor & its genomic organization. Proc Natl Acad Sci USA. 1988;85:4218-22.
- Sugino K, Kurosawa N, Nakamura T, et al. Molecular heterogeneity of follistatin, an activin-binding protein. J Biochem. 1992;68:15579-87.
- GUO Q, Kumar R, Woodruff T, et al. Overexpression of Mouse follistatin causes reproductive defects in transgenic mice. Mol Endocrinol. 1998;12:96-106.
- Robert J, Norman CR, Milner NP, et al. Circulating follistatin concentration is higher and activin concentrations are lower in polycystic ovarian syndrome. 2001;16:668-72.
- Lockwood GM, Muttukishna S, Groome NP, et al. Mid-follicular phase pulses of inhibin B are absent in polycystic ovarian syndrome and are initiated by successful laparoscopic ovarian diathermy: A possible mechanism regulating emergence of the cominent follicle. J Clin Endocrinol Metab. 1998;83: 1730-5.
- Ying S, Becker A, Swanson G, et al. Follistatin specifically inhibits pituitary follicle stimulating hormone release in vitro. Biochem Biophys Res Commun. 1987;149:133-9.
- Burger HG, Robertson DM, Farnworth PG, et al. Effects of bovine 35 kD FSH-suppressing protein on FSH & LH in rat pituitary cells in vitro: Comparison with bovine 31 kDa inhibin. J Endocrinol. 1990;124:417-23.
- DePaolo LV, Mercado M, Guo Y, et al. Increased follistatin (activin-binding protein) gene expression in rat anterior pituitary tissue after ovariectomy may be mediated by pituitary activin. Endocrinology. 1993;132:2221-8.
- Wang QF, Farnworth PG, Findlay JK, et al. Chronic inhibitory effect of follicle stimulating hormone (FSH) suppressing protein (FSP) or follistatin on activin and gonadotropin-releasing hormone stimulated FSH synthesis and secretion in cultured rat anterior pituitary cells. Endocrinology. 1990;127:1385-93.
- Schneyer AL, O’Neil DA, Crowley WF. Activin-binding proteins in human serum & follicular fluids. J Clin Endocrinol Metab. 1992;74:1320-24.
- Kruman LA, Woodruff TK, DeGuzman G, et al. Identification & characterization of binding proteins for inhibin & activin in human serum & follicular fluids. Endocrinology. 1993;132:431-43.
- Lockwood GM, Muttukrishna S, Groome NP, et al. Circulating inhibins and activin-A during GnRH-analogue down-regulation and ovarian hyperstimulation with recombinant FSH for in vitro fertilization embryo transfer. Clin Endocrinol. 1996;45:741–48.
- Muttukrishna S, Groome NP, Ledger WL. Gonadotrophic control of secretion of dimeric inhibins and activin A by human granulosa-luteal cells in vitro. J Asst Reprod Genet 1997a;14:566-74.
- Hasegawa Y, Miyamoto K, Abe Y, et al. Induction of follicle-stimulating hormone receptor by erythroid differentiation factor on rat granulose cells. Biochem Biophys Res Commun. 1988;156:668-74.
- Xiao S, Robertson DM, Findlay JK. Effects of activin and follicle-stimulating hormone (FSH)-suppressing protein/follistatin on FSH receptors and differentiation of cultured rat granulosa cells. Endocrinology. 1992;131:1009-16.
- Eldar-Geva T, Spitz IM, Groome NP, et al. Follistatin and activin A serum concentrations in obese and non-obese patients with polycystic ovary syndrome. Hum Reprod. 2001;16:2552-6.
- Lau CP, Ledger WL, Groome NP, et al. Dimeric inhibins and activin A in human follicular fluid and oocyte-cumulus culture medium. Hum Reprod. 1999;14:2525-30.
- Xia P. Intracytoplasmic sperm injection: Correlation of oocyte grade based on polar body, perivitelline space and cytoplasmic inclusions with fertilization rate and embryo quality. Hum Reprod. 1997;12:1750-5.
- Anita K, Ernest YN, William SY, et al. Perifollicular vascularity in poor ovarian responders during IVF.
- Eppig JJ. Regulation of mammalian oocyte maturation. In: Adashi E.Y., Leung P.C.K., eds. The ovary. New York: Raven Press, 1993;185–207.
- Silva CC, Knight PG. Modulatory actions of activin A and follistatin on the developmental competence of in vitro-matured bovine oocytes. Biol Reprod. 1998;58:558-65.
- Sadatsuki M, Tsutsumi O, Yamada R, et al. Local regulatory effects of activin A and follistatin on meiotic maturation of rat oocytes. Biochem Biophys Res Commun. 1993;196:388-95.
- Mather JP, Attie KM, Woodruff TK, et al. Activin stimulates spermatogonial proliferation in germ-sertoli cell co-cultures from immature rat testis. Endocrinology. 1990;127:3206-14.
- Woodruff TK, Battaglia J, Mather JP. Regulation of human granulosa cells by recombinant human activin A, recombinant human inhibin A. In: Momex R. laffiol C. Leclere I feds Prozress in Endocrinology. Proceedings of the Ninth International Coniress of Endocrinology Nice. Parthenon, Pearl River, New York. 1992;605-7.
- Xiao S, Robertson DM, Findlay JK. Effect of activin and follicle stimulating hormone (PSHI-suppressing protein/follistatin on FSH receptors and differentiation of cultured rat granulosa cells. Endocrinology. 1992;131:1009-16.
- Woodruff TK, Krummen L, Chen SA, et al. Pharrnacokinetic profile of recombinant human (rh) inhibin and activin in the immature rat. Endocrinology. 1993;132:725-34.
- Toshihiro F, Geralyn LM, Yisrael S, et al. Analysis of follicular fluid hormone concentrations and granulosa cell mRNA levels for the inhibin-activin follistatin system: Relation to oocyte and embryo characteristics. 2000;74:348-55.
- Fujiwara. The inhibin-activin-follistatin system in human follicles. Fertil Steril. 2000;74:12.
- Muttukrishna. Activin and follistatin in female reproduction. Mol Cell Endocrinol. 2004;225:45-56.
- Lau CP. Dimeric inhibins and activin A in human follicular fluid and oocyte–cumulus culture medium. Hum Reprod. 1999;14:2525-30.
- Farnworth PG, Thean E, Robertson DM, et al. Ovine anterior pituitary production of follistatin in vitro. Endocrinology. 1995;136:4397-406.
- John LF, Paul AB, Michael RD, et al. Evaluation of basal estradiol levels in assisted reproductive technology cycles. Fertil Steril. 2000;74:518-24.
- Laszlo FJ, Bancsi MM, Frank JM, et al. Performance of basal follicle-stimulating hormone in the prediction of poor ovarian response and failure to become pregnant after in vitro fertilization: A meta-analysis. Fertil Steril. 2003;79:1091-100.
- John LF, Andrew JL, Bradley TM, et al. A prospective assessment of the predictive value of basal antral follicles in in vitro fertilization cycles. Fertil Steril. 2003;80:350-5.
- Linda MF, David AG, Laura AS et al. Follicle-stimulating hormone and estradiol levels independently predict the success of assisted reproductive technology treatment. Fertil Steril. 2004;82:834-40.