The neuroprotective and anti-inflammatory effects of Myracrodruon urundeuva are related to inhibitions of brain inflammatory enzymes, cytokines and HDAC
2 Federal University of Piauà(UFPI), Brazil
3 Faculty of Medicine Estácio of Juazeiro do Norte (FMJ), Brazil, Email: cecÃliacoelhoxavier@my.edu
Received: 20-Oct-2017 Accepted Date: Nov 05, 2017; Published: 09-Nov-2017
Citation: Viana GS, Calou IB, Xavier CC, et al. The neuroprotective and antiinflammatory effects of Myracrodruon urundeuva are related to inhibitions of brain inflammatory enzymes, cytokines and HDAC. J Pharmacol Med Chem 2017;1(1):15-22.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Myracrodruon urundeuva (Anacardiaceae) is a Northeast Brazil species popularly used for its anti-inflammatory properties. Considering the involvement of neuroinflammation in neurodegenerative diseases, the objectives were to evaluate the neuroprotective actions of a standardized extract from M. urundeuva (SEMU) on a Parkinson´s disease (PD) model, focusing on brain inflammatory targets. Male Wistar rats were anesthetized subjected to stereotaxic surgery and a unilateral striatal 6-OHDA lesion. The animals were divided into sham-operated (SO), untreated 6-OHDA-lesioned and SEMU (20 mg/kg and 40 mg/kg, p.o., 14 days) treated groups. The SO group (Control) was subjected to the same procedures, but injected with saline. Afterwards the animals were sacrificed and their brains processed for dopamine (DA) determination (striata) and immunohistochemical assays (striata and hippocampi) for COX-2, iNOS, TNF-alpha, NF-kB and HDAC. The data were analyzed by one-way ANOVA and Tukey as the post hoc test. The results showed a reversion of striatal DA contents in the SEMU treated, relatively to the untreated 6-OHDA group. SEMU decreased the immunostaining for COX-2, iNOS, TNF-alpha and NF-kB in striata and hippocampal areas. In addition, SEMU decreased hippocampal HDAC immunostaining, shown to be affected in PD. The results point out to the potential neuroprotective actions of SEMU, and its chalcone contents may be responsible for the observed effects.
Keywords
Myracrodruon urundeuva; Cyclooxygenase; Nitric oxide synthase; TNFalpha; NF-kB; Histone deacetylase; neuroprotection; Parkinson’s disease
Background
Parkinson’s disease (PD) is the second most common neurodegenerative disorder and the first on movement disorders worldwide, afflicting about 5% of people over 85 years of age. The pathology of PD is characterized by a progressive loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc), associated instead of to the degeneration of projecting nerve, leading to the cardinal features of disease as tremors, muscular rigidity, bradykinesia, and postural and gait abnormalities [1].
Despite having been implemented in the 60’s, levodopa remains the “gold standard” anti-Parkinson therapy. However, its use leads to complications, such as highly disabling fluctuations of motor activity and dyskinesias [2,3].
All other options of treatment are merely palliative and neuroprotective therapies, but the cure of PD remains an unrealized goal. This, in part, may reflect a misunderstanding of the cause of neuronal death in PD. Among several reputed factors that contribute to PD pathogenesis, inflammatory mechanisms may play an important role [4,5]. It has been clearly demonstrated, in cerebral spinal fluid and blood of PD patients, high concentrations of pro-inflammatory cytokines, as well as enzymes associated with inflammation, such as inducible isoform of nitric oxide synthase and cyclooxygenase-2 [6]. This abnormal production of pro-inflammatory mediators by activated microglia and astrocytes may act as an environmental stressor to promote progressive degeneration of dopaminergic neurons [7]. The activated microglia may trigger an active self-perpetuating cycle of oxidative stress and chronic neuroinflammation [8]. Reactive oxygen species (ROS) act as secondary messengers capable of modifying gene expression in microglia-associated neurodegenerative diseases by activating mitogen activated protein kinases (MAPKs) and transcription factors, as nuclear factor-kB (NF-kB) [9-11]. Considering neuroinflammation and oxidative stress as some of the active or main driving forces in the neurodegenerative process of PD, the search of effective therapeutic strategies for its treatment is of paramount importance. Myracrodruon urundeuva Fr. Allemão, known as ‘aroeira do sertão’ in the Brazilian Northeastern region, belongs to the Anacardiaceae family and is widely used in popular medicine for treatments of pain and infections, especially in the genitourinary tract. Recent studies have demonstrated the neuroprotective and antioxidant effects of compounds isolated from M. urundeuva stem bark extracts [12]. Previous research showed the potent anti-inflammatory activity from M. urundeuva stem bark and we have shown that fractions rich in chalcones and catechic tannins are somewhat involved with the pharmacological activity of the plant [13,14]. Our group has previously demonstrated that the standardized extract and fractions are capable of decreasing microglia and astrocyte activations, in in vitro and in vivo experimental models of PD [15,16]. In this context, the present study was designed to evaluate the neuroprotective and antiinflammatory potential effects of a standardized extract of M. urundeuva (SEMU) on induced neuroinflammation, in a model of Parkinson’s disease in rats. The present study focused on brain inflammatory enzymes, proinflammatory cytokines, as TNF-alpha and histone deacetylases (HDACs), evaluated by immunohistochemistry assays, attempting to elucidate the SEMU’s mechanism of action.
Materials and Methods
Drugs and reagents
Ketamine (5% Vetanarcol) and xylazine (2% Kensol) were purchased from König (Santana de Parnaíba, São Paulo, Brazil). 6-hydroxydopamine was from Sigma Aldrich (MO, USA). The antibodies were for immunohistochemistry assays were from Santa Cruz Biotechnology (CA, USA) or from Merck (Darmstadt, Germany). All other reagents were of analytical grade.
Plant material
Plants were collected near the city of Iguatu (Ceará state, Brazil) and identified by Professor Afranio Fernandes, of the Department of Biology of the Federal University of Ceará (UFC). It is presently cultivated in the UFC Medicinal Plant Garden and a voucher specimen is deposited at the Prisco Bezerra Herbarium, under the number 14,999.
Preparation of the fluid extract from M. urundeuva (SEMU)
The fluid extracts were prepared from stem and leaves of young plants (around 70 days of development), which were dried in the oven at 40°C (with air circulation), according to the Brazilian Pharmacopeia in 2010 as previously described [17]. Briefly, the dried material (30 g) was grinded and submitted to two extractions. The first one used a mixture of Glycerol: Ethanol: H2O (1:6:3, v/v) for maceration of the dried material, for 6 h in a percolator, resulting in 24 mL of the extracted liquid material. The residue was submitted to a second extraction with a mixture of Ethanol: H2O (2:1, v/v), up to exhaustion. The resulting liquid was evaporated in a water bath at 60°C, until a syrup consistency. This syrup was added to the liquid material from the first extraction, the volume was completed to 30 mL with distilled water and the final mixture was filtered [17,18]. The stem present as characteristic chemical markers dimeric chalcones such as urundeuvines A and B (Figure 1) isolated by preparative chromatography on a corn starch column. All the compounds had their spectral data identified by 13C NMR (Table 1). The stems showed by spectrometric assays 2.21% of total tannins expressed as galic acid equivalent.
| C | Urundeuvine A (I) | Urundeuvine B (II) |
|---|---|---|
| 1 | 124.85 | 129.37 |
| 2 | 131.40 | 130.17 |
| 4 | 148.71 | 150.01 |
| 5 | 145.22 | 149.30 |
| 8 | 124.85 | 130.31 |
| 9 | 199.22 | 200.67 |
| 1’ | 113.39 | 114.20 |
| 2’ | 165.85 | 166.70 |
| 4’ | 166.97 | 167.14 |
| 1” | 134.66 | 132.41 |
| 4” | 157.30 | 157.76 |
| 7” | - | 137,80 |
| 8” | - | 133.46 |
| 9” | 202.66 | 202.66 |
| 1”’ | 113.06 | 116.29 |
| 2”’ | 165.70 | 166.19 |
| 4”’ | 166.40 | 165.69 |
| CH | ||
| 3 | 116.85 | 109.79 |
| 6 | 117.31 | 111.79 |
| 7 | 141.65 | 128.66 |
| 3’ | 103.91 | 103.70 |
| 5’ | 108.57 | 108.89 |
| 6’ | 135.66 | 137.38 |
| 2”,6” | 130.17 | 132.83 |
| 3”,5” | 116.30 | 115.68 |
| 7” |  48.39 | - |
| 8” |  51.12 | - |
| 3”’ | 103.80 | 102.91 |
| 5”’ | 108.96 | 108.30 |
| 6”’ | 133.99 | 137.01 |
Table 1: 13C NMR spectral data of urundeuvine A (I) and urundeuvine B (II) isolated from M. urundeuva stem. The chemical displacements are described in δ (ppm)
Animals
Male Wistar rats weighing approximately 250 g from the Animal House of the Faculty of Medicine Estácio of Juazeiro do Norte (Ceará, Brazil) were divided into six groups (SO, untreated 6-OHDA and 6-OHDA treated with SEMU, at the doses of 20 and 40 mg/kg, p.o.), ranging from 5 to 9 animals.
The experimental protocol followed the one previously described (16). The doses were chosen based on a previous work (16). The treatments were carried by gavage (orogastric tube) and started 1 h after the stereotaxic surgery, continuing daily for 14 days. The sham-operated (SO) which represents the control group and the untreated 6-OHDA group were also orally administered with distilled water, under the same experimental conditions as those of the 6-OHDA groups treated with SEMU. The animals were maintained in plastic cages, with food and water ad libitum, on a 12 h/12 h light/dark cycle, at a 23°C temperature. All experiments were carried out according to the Guide for the Care and Use of Laboratory Animals, USA, 2011. The project was approved by the Ethics Committee on Animals Experimentation of the Faculty of Medicine of the Federal University of Ceará, under the number 43/13.
Experimental model of Parkinson’s disease (PD)
This model consists of a unilateral intrastriatal infusion of 6-OHDA and involves a massive destruction of nigrostriatal dopaminergic neurons. It is largely used for investigating motor and biochemical dysfunctions in Parkinson’s disease [19]. This model resembles the key features of human parkinsonian gait [20] and is known to induce a nigrostriatal lesion in rodents. In addition, it is of low complexity and cost, and the 6-OHDAinduced lesion is highly reproducible, yielding degrees of nigrostriatal lesions that develop with different temporal profiles, depending upon the site of the neurotoxin injection [21]. In the present study, the animals were anesthetized with ketamine (80 mg/kg, i.p.) and xylazine (20 mg/kg, i.p.) and the stereotaxic surgery was performed as previosly described [16].
Dopamine (DA) contents determination by HPLC
We used the procedure previously described by Correia et al., 2016. Briefly, the right lesioned striata were utilized for preparing 10% homogenates in 0.1 M HClO4, that were sonicated (30 s) and centrifuged at 4°C (15,000 rpm, 15 min). The supernatants were filtered and 20 μL injected into the HPLC column (Shim-Pak CLC-ODS, 25 cm), with a flow of 0.6 mL/ min and coupled to an electrochemical detector (model L-ECD-6A, from Shimadzu, Japan). A mobile phase was prepared with monohydrated citric acid (150 mM), sodium octyl sulfate (67 mM), 2% tetrahydrofuran and 4% acetonitrile, in deionized water and the final pH adjusted to 3.0. DA was quantified by comparison with standards and the results expressed as ng/g tissue.
Immunohistochemistry for brain COX-2, iNOS, TNF-alpha, NF-kB and HDAC
The loss of dopaminergic neurons and neuroinflammation shown in PD is a chronic process, associated with a glial response composed of activated glial cells, including astrocytes and microglia [22,23]. These assays were performed, as previously described [16], except for the primary antibodies. Thus, in the present study, TNF-alpha, COX-2, iNOS and NF-kB monoclonal antibodies were from Santa Cruz Biotecnology (CA, USA). The HDAC antibody (rabbit polyclonal IgG) was from Merck KGaA (Darmstadt, Germany). All antibodies were diluted according the manufacturers’ instructions. The data from 3 animals were analyzed by the Image J software (NIH, USA) in the Nikon Eclipse Ni microscope coupled to the DS Ri2 camera (for the capture of the entire image area). The results were expressed as optical density per field.
Statistical analysis
The results are expressed as means ± SEM. For multiple comparisons, the data were analyzed by one-way ANOVA, followed by Tukey as the post hoc test and significant results presented as q (the studentized range statistics) and p values. The results from the immunohistochemical assays were quantified by the Image J software (NIH, USA) and also analyzed by one-way ANOVA, followed by Tukey as the post hoc test.
Results
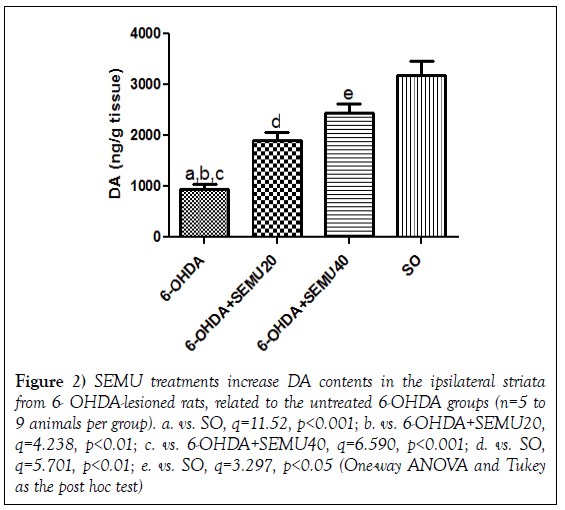
DA contents in 6-OHDA lesioned striata in the untreated 6-OHDA groups and in SEMU treated groups (6-OHDA+SEMU20 and 6-OHDA+SEMU40). (Figure 2) shows that the 6-OHDA lesion (right striatum) causes a 70% reduction in DA contents, in relation to the SO group. Smaller decreases were demonstrated in lesioned right striata of the 6-OHDA groups, after SEMU treatments with the doses of 20 (40% decreases) and 40 mg/kg (23% decreases). These results point out to the neuroprotection afforded by SEMU treatments, in this PD model.
Figure 2: SEMU treatments increase DA contents in the ipsilateral striata from 6- OHDA-lesioned rats, related to the untreated 6-OHDA groups (n=5 to 9 animals per group). a. vs. SO, q=11.52, p<0.001; b. vs. 6-OHDA+SEMU20, q=4.238, p<0.01; c. vs. 6-OHDA+SEMU40, q=6.590, p<0.001; d. vs. SO, q=5.701, p<0.01; e. vs. SO, q=3.297, p<0.05 (One-way ANOVA and Tukey as the post hoc test)
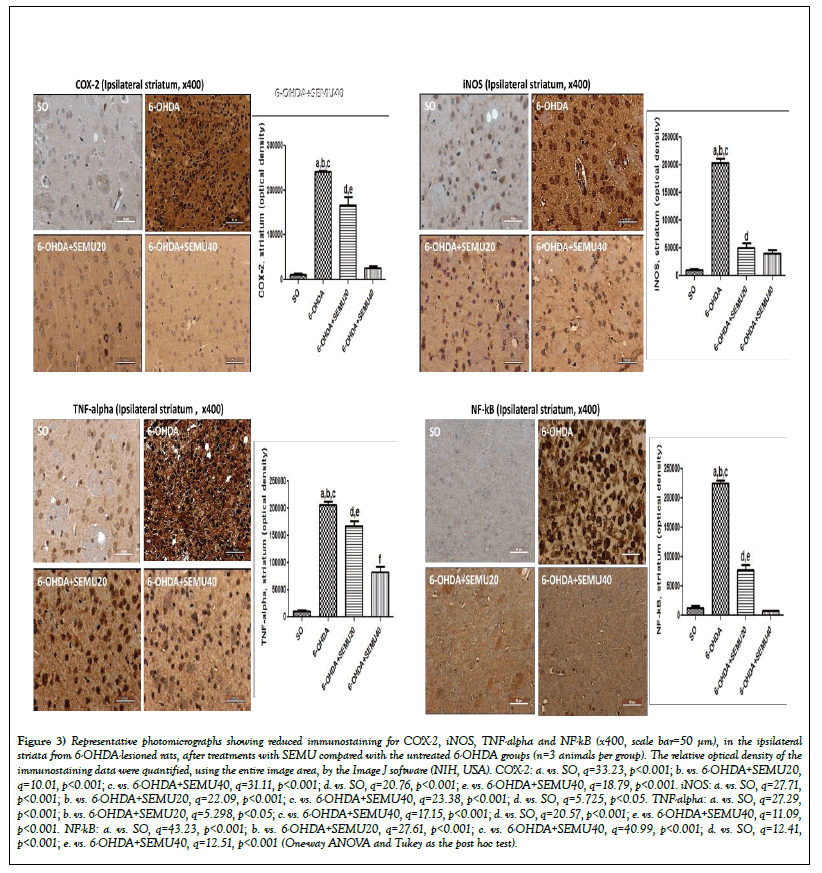
Immunohistochemistry for COX-2, iNOS, TNF-alpha and NF-kB in striata from 6-OHDA-lesioned groups, before (untreated 6-OHDA groups) and after SEMU treatments. Immunostaining for COX-2 revealed a 21-times increase in the untreated 6-OHDA group, compared with the SO group. However, this value was 15- and 2-times lower after SEMU treatments with the doses of 20 and 40 mg/kg, respectively (Figure 3A). A 19-times increase was observed for iNOS immunohistochemistry in the untreated 6-OHDA-lesioned group, related to the SO group, while 5- and 4-times increases were observed after SEMU treatments (Figure 3B). Similar results were demonstrated, in the 6-OHDA-lesioned striata, for TNF-alpha immunostaining (19-times increase). However, the effects were lower after SEMU20 (16-times decrease) and SEMU40 (8-times decrease) (Figure 3C). Increases of 18-times were demonstrated in NF-kB immunostaining in untreated 6-OHDA-lesioned groups, compared with the SO group. On the other hand, decreases of 6- and 1.7-times were observed after SEMU treatments with the doses of 20 and 40 mg/kg, respectively (Figure 3D).
Figure 3: Representative photomicrographs showing reduced immunostaining for COX-2, iNOS, TNF-alpha and NF-kB (x400, scale bar=50 μm), in the ipsilateral striata from 6-OHDA-lesioned rats, after treatments with SEMU compared with the untreated 6-OHDA groups (n=3 animals per group). The relative optical density of the immunostaining data were quantified, using the entire image area, by the Image J software (NIH, USA). COX-2: a. vs. SO, q=33.23, p<0.001; b. vs. 6-OHDA+SEMU20, q=10.01, p<0.001; c. vs. 6-OHDA+SEMU40, q=31.11, p<0.001; d. vs. SO, q=20.76, p<0.001; e. vs. 6-OHDA+SEMU40, q=18.79, p<0.001. iNOS: a. vs. SO, q=27.71, p<0.001; b. vs. 6-OHDA+SEMU20, q=22.09, p<0.001; c. vs. 6-OHDA+SEMU40, q=23.38, p<0.001; d. vs. SO, q=5.725, p<0.05. TNF-alpha: a. vs. SO, q=27.29, p<0.001; b. vs. 6-OHDA+SEMU20, q=5.298, p<0.05; c. vs. 6-OHDA+SEMU40, q=17.15, p<0.001; d. vs. SO, q=20.57, p<0.001; e. vs. 6-OHDA+SEMU40, q=11.09,p<0.001. NF-kB: a. vs. SO, q=43.23, p<0.001; b. vs. 6-OHDA+SEMU20, q=27.61, p<0.001; c. vs. 6-OHDA+SEMU40, q=40.99, p<0.001; d. vs. SO, q=12.41, p<0.001; e. vs. 6-OHDA+SEMU40, q=12.51, p<0.001 (One-way ANOVA and Tukey as the post hoc test).
Immunohistochemistry for COX-2, iNOS and TNF-alpha in hippocampi from 6-OHDA-lesioned groups, before (untreated 6-OHDA) and after SEMU treatments. Immunostaining for COX-2 increased by 7-times in the CA3 subfield; in untreated 6-OHDA groups compared with the SO group. Five- and only 2-times increases were observed in this region, after SEMU treatments with the doses of 20 and 40 mg/kg, respectively (Figure 4A). The untreated 6-OHDA group showed a high immunostaining for iNOS in the CA3 area, compared with the SO group (almost 300%). This value went down to 206% and to only 35% after SEMU treatments with the doses of 20 and 40 mg/kg, respectively (Figure 4B). The neurotoxin 6-OHDA also increased by 28-times TNF-alpha levels in the CA3 hippocampal subfield, compared with the SO group. This increase was of only 10-times in the 6-OHDA group after SEMU treatment with the dose of 40 mg/kg (Figure 4C).
Figure 4: Representative photomicrographs showing reduced immunostaining for COX-2, iNOS and TNF-alpha (x400, scale bar=50 μm), in CA3 areas from 6-OHDAlesioned rats after SEMU treatments, compared to the untreated 6-OHDA groups (3 animals per group). The data were quantified, using the entire image area, by the Image J software (NIH, USA). COX-2: a. vs. SO, q=15.21, p<0.001; b. vs. 6-OHDA+SEMU20, q=5.924, p<0.01; c. vs. 6-OHDA+SEMU40, q=12.97, p<0.001; d.vs. SO, q=8.162, p<0.001; e. vs. 6-OHDA+SEMU40, q=6.082, p<0.01. iNOS: a. vs. SO, q=62.57, p<0.001; b. vs. 6-OHDA+SEMU20, q=17.79, p<0.001; c. vs.6-OHDA+SEMU40, q=55.42, p<0.001; d. vs. SO, q=40.14, p<0.001; e. vs. 6-OHDA+SEMU40, q=33.52, p<0.001; f. vs. SO, q=7.155, p<0.01. TNF-alpha: a. vs. SO, q=95.89, p<0.001; b. vs, 6-OHDA+SEMU40, q=65.10, p<0.001; c. vs. SO, q=30.74, p<0.001 (one-way ANOVA and Tukey as the post hoc test).
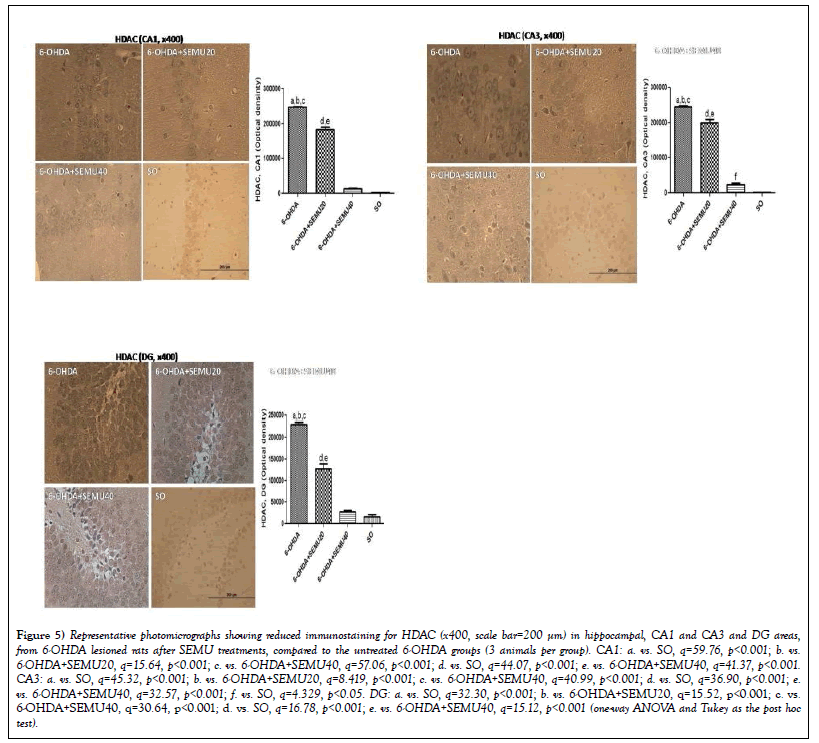
Immunohistochemistry for histone deacetylase (HDAC) in hippocampi and temporal cortices from 6-OHDA-lesioned groups, before (untreated 6-OHDA) and after SEMU treatments. HDACs are known to reduce gene expression and regulate protein clearance. Inhibitors of HDACs have been reported to be potentially efficacious in neuredegenerative pathologies, including Parkinson’s disease [24-26]. We showed a 94-times increase in immunostaining for HDAC in the CA1 subfield, in 6-OHDA lesioned animals, related to the SO groups. This value decreased to 7- and 5-times in the 6-OHDA lesioned group, after the treatments with SEMU, 20 and 40 mg/kg (Figure 5A). Similar, although higher values were observed in the CA3 subfield, with increases of 197-times in untreated 6-OHDA-lesioned groups. These values dropped to 60- and 20-times increases after treatments with SEMU at the doses of 20 and 40 mg/kg, respectively (Figure 5B). The untreated 6-OHDA lesioned group presented increases in HDAC immunostaining (14-times increase) in the dentate gyrus, compared to the SO groups, while SEMU treatments, at the doses of 20 and 40 mg/kg, showed only 8- and 2-fold increases, respectively (Figure 5C).
Figure 5: Representative photomicrographs showing reduced immunostaining for HDAC (x400, scale bar=200 μm) in hippocampal, CA1 and CA3 and DG areas, from 6-OHDA lesioned rats after SEMU treatments, compared to the untreated 6-OHDA groups (3 animals per group). CA1: a. vs. SO, q=59.76, p<0.001; b. vs. 6-OHDA+SEMU20, q=15.64, p<0.001; c. vs. 6-OHDA+SEMU40, q=57.06, p<0.001; d. vs. SO, q=44.07, p<0.001; e. vs. 6-OHDA+SEMU40, q=41.37, p<0.001.CA3: a. vs. SO, q=45.32, p<0.001; b. vs. 6-OHDA+SEMU20, q=8.419, p<0.001; c. vs. 6-OHDA+SEMU40, q=40.99, p<0.001; d. vs. SO, q=36.90, p<0.001; e.vs. 6-OHDA+SEMU40, q=32.57, p<0.001; f. vs. SO, q=4.329, p<0.05. DG: a. vs. SO, q=32.30, p<0.001; b. vs. 6-OHDA+SEMU20, q=15.52, p<0.001; c. vs.6-OHDA+SEMU40, q=30.64, p<0.001; d. vs. SO, q=16.78, p<0.001; e. vs. 6-OHDA+SEMU40, q=15.12, p<0.001 (one-way ANOVA and Tukey as the post hoc test).
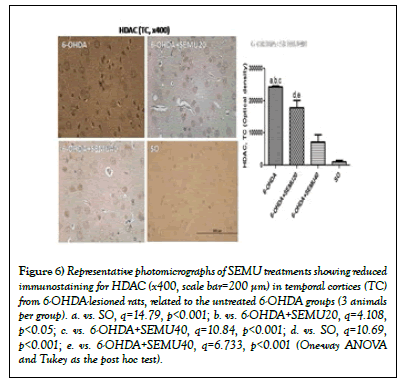
In addition, a 23-times increase in HDAC immunostaining was shown in TC, in the untreated 6-OHDA lesioned groups, compared with the SO group. This value decreased to 17- and to 7-times after treatments with SEMU, at the doses of 20 and 40 mg/kg (Figure 6).
Figure 6: Representative photomicrographs of SEMU treatments showing reduced immunostaining for HDAC (x400, scale bar=200 μm) in temporal cortices (TC) from 6-OHDA-lesioned rats, related to the untreated 6-OHDA groups (3 animals per group). a. vs. SO, q=14.79, p<0.001; b. vs. 6-OHDA+SEMU20, q=4.108, p<0.05; c. vs. 6-OHDA+SEMU40, q=10.84, p<0.001; d. vs. SO, q=10.69, p<0.001; e. vs. 6-OHDA+SEMU40, q=6.733, p<0.001 (One-way ANOVA and Tukey as the post hoc test).
Discussion
In this study, we investigated potential anti-neuroinflammatory effects of a standardized extract from Myracrodruoun urundeuva in hemi-parkinsonian rats. Neurotoxin-based animal models have largely been used to induce selective neuronal death, in both in vitro and in vivo studies. 6-Hydroxydopamine (6- OHDA) is the most reproduced model of Parkinson’s disease, inducing a rapid and pronounced dopaminergic neurodegeneration with the associated motor dysfunction [27]. The toxin initiates dopaminergic neurons (DA) degeneration through a combination of oxidative stress and mitochondrial dysfunction, making the tool suitable for an effective model [28]. Oxidative stress activates microglia and leads to neurotoxicity of DA neurons [29].
As the treatment of PD focuses on symptomatic relief, rather than the improvement of pathogenesis progression, the discovery of triggering pathways is of great importance in the search for effective therapeutic strategies. It is well established the relationship between severity of motor dysfunction and the seletive loss of dopamine neurons, what is at least in part associated with severe oxidative stress and inflammation [30,31]. In this context, the development of disease-modifying agents based on the use of drugs with antioxidant and anti-inflammatory actions, along with other pharmacological properties, would be useful for retarding the development and progression of PD [32,33].
Thus, oxidative injury and neuroinflammation contribute significantly to PD pathogenesis. In order to investigate alternative and early intervention approaches to prevent or halt the progressive nature of PD, we evaluated the potential of SEMU in 6-OHDA-induced neurodegeneration. With central nervous system activities yet poorly studied, M. urundeuva has welldefined anti-inflammatory and antioxidant actions, mainly attributed to its content of phenolic compounds [13,34,35]. Based on these findings, we demonstrated that chalcones from M. urundeuva exerted neuroprotection, on rat mesencephalic cells, by reducing the oxidative stress and apoptotic injury caused by 6-OHDA. Subsequently, the neuroprotective activity of the standardized extract of M. urundeuva was confirmed, in vivo, by inhibitions of microglia and astrocyte activation [15,16].
PET analysis showed that PD patients, with or without dementia, present significant microglial activation in cortical brain regions, suggesting that neuroinflammation could occur early in PD, persisting as the disease progresses [36]. Insults to the central nervous system (CNS) triggers microglial activation, leading to the recruitment of peripheral leukocytes. Furthermore, inflammatory mediators not only modulate immune cells, but also contribute to neurodegeneration, resulting in a vicious cycle of inflammation and neuronal death [7]. Thus, targeting microglial activation appears as a valid therapeutic strategy for PD treatment [37].
Our results show that 6-OHDA caused in the striatum an increase in immunohistochemical labeling of inflammatory mediators, such as TNFalpha, COX-2, iNOS, as well as the transcription factor NF-kB, whereas its immunostaining was restored to the normal range after the SEMU treatment. The effects of 6-OHDA are in agreement with other studies that show the activation of microglia and subsequent increase of the overexcited mediators to be a major cause of the neurotoxin effects [38,39]. Activation of NF-kB has been linked to oxidative stress-induced apoptosis in PD and seems to be involved in regulating inflammatory responses in microglial cells [40].
The TNF-alpha signaling pathway induces upregulation of inflammatory mediators involved in apoptotic cells, including caspase-1, caspase-3, and TNF-alpha receptor R1. This pro-inflammatory cytokine has already been identified in the substantia nigra (SN) from Parkinsonian patients, indicating the occurrence of a proapoptotic environment in PD [41]. Prolonged TNFalpha expression induced in the SN of adult rats leads to dopaminergic neuronal death, motor symptoms and microglia activation [42]. Furthermore, inhibition of NF-κB attenuates production of TNF-alpha and other glial derived inflammatory mediators, thus reducing the neurotoxicity of activated microglial cultures on dopaminergic neuron-like cells [43].
Accordingly, the effects observed with SEMU may be exerted through inhibition of the NF-kB activation in DA neurons, inducing down-regulation of COX-2 in the brain that could subsequently decrease the release of TNFalpha. Inhibition of COX-mediated DA oxidation [44], as well as microglialderived toxic mediator production are likely to be among the mechanisms that contribute to decreased incidence of PD in chronic NSAID users [45]. A non-selective COX-2 inhibitor has shown to present a neuroprotective activity in the 6-OHDA rat model of PD [46], what is consistent with the neuroinflammatory hypothesis for PD pathogenesis. The activation of NF- κB also promotes the induction of iNOS from activated microglia [6]. This, in turn, increases the production of NO that reacts with superoxide anion and generates peroxynitrite, a highly reactive molecule, causing striatal neurodegeneration in the 6-OHDA model of PD [47]. In our study, evidence for the anti-inflammatory activity of SEMU is further supported by the attenuation of iNOS striatal immunostaining, what can be explained by its NF-κB inhibitory activity.
6-OHDA induces selective loss of nigroestriatal neurons through oxidative stress, mitochondrial inhibition, iron accumulation and inflammation [48]. Normally, iNOS is not expressed in the brain, unless under pathological situations, especially those associated with gliosis. Once expressed, iNOS produces high and continuous levels of NO from microglia or astrocytes [49]. We observed that animals treated with SEMU showed a significative decrease in immunostaining for iNOS, in the striatum. Therefore, through inhibition of glial activation-mediated oxidative stress, by reducing iNOS, the tested extract has therapeutic value in the treatment of neuroinflammation related to PD.
The cellular and molecular components of the innate inflammatory response, associated with a slowly progressive degenerative disease, are not clearly identified. Nevertheless, NF-κB, known as the major transcriptional factor of a wide range of cytokines, has been extensively associated with neuronal signaling and stress response [50]. Activation of the NF-κB pathway has been reported to be as much as 70-fold higher in the SN of PD patients, compared to that of age-matched healthy controls [51]. An inhibitor of NF-κB attenuates the production of TNF-alpha and other glial derived inflammatory mediators, thereby reducing the neurotoxicity of activated microglial cultures on dopaminergic neuron-like cells [43]. Besides that, the NF-κB pathway contributes to the initiation of oxidative stress [52], what may explain the sensitive effect of SEMU on neuroprotection through antiinflammatory and antioxidant actions.
Besides decreasing striatal immunostaining for inflammatory enzymes, TNFalpha and NF-kB, SEMU neuroprotection is also afforded by the inhibition of these same targets on the hippocampus. Although a main feature of PD is dopaminergic loss in the SNpc, evidences are accumulating on hippocampal alteration, what would be responsible for some of non-motor characteristics of this disease [53-56]. In the present work, we demonstrated that SEMU significantly decreased the immunostaining for iNOS, COX-2 and TNFalpha in the hippocampal CA3 subfield. Similar results were observed in the dentate gyrus. The structural brain changes in PD seem to be present mainly in those patients with dementia [54] and memory deficits [55]. Although cortical areas are involved, the hippocampus is one of the regions most affected in PD, with decreasing grey matter volume, pointing out to the potential of hippocampal atrophy as a structural biomarker in PD [56].
Furthermore, for the first time, we demonstrated that SEMU is also an inhibitor of brain histone deacetylases (HDACs). HDACs are a family of enzymes that catalize the removal of acetyl groups from lysine residues of proteins. It is largely accepted that acetylation and deacetylation of histone proteins associated with chromatin are crucial in the epigenetic regulation of transcription and other cellular functions [57]. Furthermore, HDACs have received increasing attention in the context of neurological diseases and appear as therapeutic targets in neurodegeneration [58]. Interestingly, the possible action mechanisms for the neuroprotective action of HDAC inhibitors may involve transcriptional activation of neuronal survival genes and maintenance of histone acetylation homeostasis that are dysregulated in PD [26].
More seletive HDAC inhibitors have been shown to protect neuronal cells from death and may be potential therapeutic agents against neurotoxicity [59]. Animal model studies of neurodegenerative pathologies emphasize the potential role of epigenetic drugs, as HDAC inhibitors, in ameliorating the cognitive and motor symptoms of PD [24]. A recent work [60] revealed that PD environmental factors induced HDACs degradation and histone acetylation increase in DA neurons, via autophagy, pointing out to an epigenetic mechanism in PD pathogenesis. Furthermore, HDAC inhibitors are also anti-inflammatory drugs [61-63]. Interestingly, natural chalcones were observed to present a dual inhibiton of both HDACs and NF-kB [64]. Chalcones are also major bioactive components present in M. urundeuva, as already shown by us [13].
Conclusion
In conclusion, for the first time, this study demonstrated that a standardized extract from M. urundeuva is able to reduce the inflammatory environmental consequence of microglial activation induced by 6-OHDA dopaminergic neurodegeneration, through inhibition of inflammatory enzymes, NF-KB as well as HDAC. Most importantly, taken together, the present findings and those from previous reports indicate that SEMU appears to be a promising agent of natural origin for protection against PD and neurodegeneration.
Acknowledgements
The authors are grateful to the financial support from the Brazilian National Research Council (CNPq) and the Foundation for Support of Scientific and Technological Development of the State of Ceará, Brazil (FUNCAP). They also thank the manuscript ortographic revision by Prof. M.O.L. Viana.
Conflict of Interest
The authors declare no conflict of interests.
REFERENCES
- Kowal SL, Dall TM, Chakrabarti R, et al. The current and projected economic burden of Parkinson's disease in the United States. Mov Disord 2013;28:311-8.
- Sharma S, Singh S, Sharma V, et al. Neurobiology of l-DOPA induced dyskinesia and the novel therapeutic strategies. Biomed Pharmacother 2015;70:283-93.
- Giugni JC, Okun MS. Treatment of advanced Parkinson's disease. Curr Opin Neurol 2014;27:450-60.
- Glass CK, Saijo K, Winner B, et al. Mechanisms underlying inflammation in neurodegeneration. Cell 2010;140:918-34.
- Wang Q, Liu Y, Zhou J. Neuroinflammation in Parkinson’s disease and its potential as therapeutic target. Transl Neurodegen 2015;12;4:19.
- Knott C, Stern G, Wilkin GP. Inflammatory regulators in Parkinson’s disease: iNOS, lipocortin-1, and cyclooxygenases-1 and -2. Mol Cell Neurosci 2000;16:724-39.
- Rocha NP, De Miranda AS, Teixeira AL. Insights into neuroinflammation in Parkinson's disease: From biomarkers to anti-inflammatory based therapies. Biomed Res Int 2015;1-12.
- Pontiki E, Kontogiorgis C, Xu Y, et al. New lipoxygenase inhibitors of reactive oxygen species production in cellular models of amyloid (A2) toxicities. J Alzheimers Dis 2012;34:215-30.
- Pawate S, Shen Q, Fan F, et al. Redox regulation of glial inflammatory response to lipopolysaccharide and interferon gamma. J Neurosci Res 2004;77:540-551.
- Flood PM, Qian Li, Peterson LJ, et al. Transcriptional factor NF-kB as a target for therapy in Parkinson’s disease. Parkinson’s disease 2011;1-8
- Blesa J, Trigo-Damas I, Quiroga-Varela A, et al. Oxidative stress and Parkinson’s disease. Front Neuroanat 2015;9:91.
- Souza LP. Standardization of plant extracts: Astronium urundeuva (Anacardiaceae). Dissertation, Institute of Chemistry, Universidade Estadual Paulista, Araraquara, Brazil 2012.
- Viana GSB, Bandeira MAM, Matos FJA. Analgesic and anti-inflammatory effects of chalcones isolated from Myracrodruon urundeuva Allemão. Phytomedicine 2003;10:189-95.
- Souza TM, Farias DF, Soares BM, et al. Toxicity of Brazilian plant seed extracts to two strains of Aedes aegypti (Diptera: Culicidae) and non-target animals. J Med Entomol 2011;48:846-51.
- Nobre-Júnior HV, Oliveira RA, Maia FD, et al. Neuroprotective effects of chalcones from Myracrodruon urundeuva on 6-hydroxydopamine-induced cytotoxicity in rat mesencephalic cells. Neurochem Res 2009;34:1066-75.
- Calou I, Bandeira MA, Aguiar-Galvão W, et al. Neuroprotective properties of a standardized extract from Myracrodruon urundeuva Fr. All. (Aroeira-Do-Sertão), as evaluated by a Parkinson’s disease model in rats. J Parkinsons Dis 2014;1-11.
- Aguiar WR. Development of pharmaceutical techniques for obtaining plant drugs from buds and renewals of Myracrodruon urundeuva Allemão (Aroeira-do-Sertão). Dissertation. Pharmaceuticals Science. Federal University of Ceará, Brazil 2013;102.
- Bandeira MAN. Myracrodruon urundeuva Allemão (aroeira-do-sertão): Active chemical constituents of the plant in development and adult. Ph.D. Dissertation in Organic Chemistry. Federal University of Ceará, Brazil 2002;322.
- Blandini F, Armentero MT, Martignoni E. The 6-hydroxydopamine model: news from the past. Parkinsonism Relat Disord 2008;14:124-9.
- Metz GA, Tse A, Ballermann M, et al. The unilateral 6 OHDA rat model of Parkinson’s disease revisited: an electromyographic and behaviour analysis. Eur J Neurosci 2005;22:735-44.
- Simola N, Morelli M, Carta AR. The 6-hydroxydopamine model of Parkinson’s disease. Neurotox Res 2007;11:151-67.
- Lull ME, Block ML. Microglial activation and chronic neurodegeneration. Neurotherapeutics 2010;7:354-365.
- More SV, Kumar H, Kim IS, et al. Cellular and molecular mediators of neuroinflammation in the pathogenesis of Parkinson’s disease. Med Inflamm 2013;1-12.
- Coppedè F. The potential of epigenetic therapies in neurodegenerative diseases. Front Genet 2014;14:220.
- Akela MA, Saki SA, Ebrahim WM, et al. Immunohistochemical studies on HDAC-4 in experimentally induced Parkinson’s diseases. Am J Biol Life Sci 2015;3:65-74.
- Sharma S, Taliyan R. Targeting histone deacetylases: A novel approach in Parkinson’s disease. Parkinsons Dis 2015;1-11
- Naughton C, Moriarty N, Feehan J, et al. Differential pattern of motor impairments in neurotoxic, environmental and inflammation-driven rat models of Parkinson’s disease. Behav Brain Res 2016;296:451-458.
- Didonet JJ, Cavalcante JC, Souza LS, et al. Neuropeptide S counteracts 6-OHDA-induced motor deficits in mice. Behav Brain Res 2014;266:29-36.
- Haddadi R, Mohajjel NA, Shahla EB. Pre-treatment with silymarin reduces brain myeloperoxidase activity and inflammatory cytokines in 6-OHDA hemi-parkinsonian rats. Neurosci Lett 2013;555:106-111.
- Dexter DT, Jenner P. Parkinson disease: from pathology to molecular disease mechanisms. Free Radic Biol Med 2013;62:132-44.
- Niranjan R. The role of inflammatory and oxidative stress mechanisms in the pathogenesis of Parkinson’s disease: Focus on astrocytes. Mol Neurobiol 2014;49:28-38.
- Al-Dakheel A, Beaulieu-Boire I, Fox SH. Emerging drugs for levodopa-induced dyskinesia. Expert Opin Emerg Drugs 2014;19:415-29.
- Fu MH, Li CL, Lin HL, et al. Stem cell transplantation therapy in Parkinson’s disease. Springer Plus 2015;4:597.
- Viana GSB. Aroeira-do-sertão: Botanical, pharmacognostic, chemical and pharmacological study. Fortaleza: Universidade Federal do Ceará 1995;164.
- Viana GSB, Bandeira MAM, Moura LC, et al. Analgesic and anti-inflammatory effects of the tannin fraction from Myracrodruon urundeuva Fr. All. Phytother Res 1997;11:118-22.
- Edison P, Ahmed I, Fan Z, et al. Microglia, amyloid, and glucose metabolism in Parkinson's disease with and without dementia. Neuropsychopharmacol 2013;38:938-49.
- Subramaniam SR, Federoff HJ. Targeting microglial activation states as a therapeutic avenue in Parkinson’s disease. Front Aging Neurosci 2017;9:176.
- Mogi M, Togari A, Tanaka K, et al. Increase in level of tumor necrosis factor (TNF)-a in 6-hydroxydopamine-lesioned striatum in rats without influence of systemic l-DOPA on the TNF-a induction. Neurosci Lett 1999;:101–4.
- Okuno T, Nakatsuji Y, Kumanogoh A, et al. Loss of dopaminergic neurons by the induction of inducible nitric oxide synthase and cyclooxygenase-2 via CD 40: Relevance to Parkinson's disease. J Neurosci Res 2005;81:874-82.
- Rosenstiel P, Lucius R, Deuschl G, et al. From theory to therapy: Implications from an in vitro model of ramified microglia. Microsc Res Tech.2001;54:18-25.
- Mogi M, Togari A, Kondo T, et al. Caspase activities and tumor necrosis factor receptor R1 (p55) level are elevated in the substantia nigra from Parkinsonian brain. J Neural Transm 2000; 107:335-41.
- De Lella Ezcurra AL, Chertoff M, Ferrari C, et al. Chronic expression of low levels of tumor necrosis factor-alpha in the Substantia nigra elicits progressive neurodegeneration, delayed motor symptoms and microglia/macrophage activation. Neurobiol Dis 2010;37:630-40.
- Tran TA, McCoy MK, Sporn MB, et al. The synthetic triterpenoid CDDO-methyl ester modulates microglial activities, inhibits TNF production, and provides dopaminergic neuroprotection. J Neuroinflammation 2008;5:14.
- Teismann P, Vila M, Choi DK, et al. COX-2 and neurodegeneration in Parkinson's disease. Ann N Y Acad Sci 2003;991:272-7.
- Tansey MG, Goldberg MS. Neuroinflammation in Parkinson's disease: Its role in neuronal death and implications for therapeutic intervention. Neurobiol Dis 2010;37:510-8.
- Sánchez-Pernaute R, Ferree A, Cooper O, et al. Selective COX-2 inhibition prevents progressive dopamine neuron degeneration in a rat model of Parkinson's disease. J Neuroinflammation 2004;1:6.
- Mihm MJ, Schanbacher BL, Wallace BL, et al. Free 3-nitrotyrosine causes striatal neurodegeneration in vivo. J Neurosci 2001;21:RC149.
- Broom L, Marinova-Mutafchieva L, Sadeghian M, et al. Neuroprotection by the selective iNOS inhibitor GW274150 in a model of Parkinson disease. Free Radic Biol Med. 2011;50:633-40.
- Bal-Price A, Brown GC. Inflammatory neurodegeneration mediated by nitric oxide from activated glia-inhibiting neuronal respiration, causing glutamate release and excitotoxicity. J Neurosci. 2001;21:6480-91.
- Nair VD, Ge Y. Alterations of miRNAs reveal a dysregulated molecular regulatory network in Parkinson's disease striatum. Neurosci Lett. 2016;26:99-104
- Hunot S, Brugg B, Ricard D, et al. Nuclear translocation of NF-kappaB is increased in dopaminergic neurons of patients with Parkinson disease. Proc Natl Acad Sci USA. 1997;94:7531-6.
- Zhao D, Yang J, Yang L. Insights for oxidative stress and mTOR sigbaling in myocardial ischemia/reperfusion injury under diabetes. Oxidative Medicine and Cellular Longevity. 2017;1-12.
- Burton EJ, McKeith IG, Burn DJ, et al. Cerebral atrophy in Parkinson's disease with and without dementia: A comparison with Alzheimer's disease, dementia with Lewy bodies and controls. Brain 2004;127:791-800.
- Summerfield C, Junqué C, Tolosa E, et al. Structural brain changes in Parkinson disease with dementia: a voxel-based morphometry study. Arch Neurol 2005;62:281-5.
- Carlesimo GA, Piras F, Assogna F, et al. Hippocampal abnormalities and memory deficits in Parkinson disease: A multimodal imaging study. Neurology 2012;78:1939-45.
- Apostolova L, Alves G, Hwang KS, et al. Hippocampal and ventricular changes in Parkinson's disease mild cognitive impairment. Neurobiol Aging 2012;33:2113-24.
- Chuang DM, Leng Y, Marinova Z, et al. Multiple roles of HDAC inhibition in neurodegenerative conditions. Trends Neurosci 2009;32:591-60.
- Didonna A, Opal P. The promise and perils of HDAC inhibitors in neurodegeneration. Ann Clin Transl Neurol 2015;2:79-101.
- Durham B. Novel histone deacetylase (HDAC) inhibitors with improved selectivity for HDAC2 and 3 protect against neural cell death. Biosci Horiz 2012;5:hzs003.
- Park G, Tan J, Garcia G, et al. Regulation of histone acetylation by autophagy in Parkinson disease. J Biol Chem 2016;291:3531-40.
- Adcock IM. HDAC inhibitors as anti-inflammatory agents. Br J Pharmacol 2007;150:829-31.
- Halili MA, Andrews MR, Sweet MJ, et al. Histone deacetylase inhibitors in inflammatory disease. Curr Top Med Chem 2009;9:309-19.
- Grabiec AM, Tak PP, Reedquist KA. Function of histone deacetylase inhibitors in inflammation. Crit Rev Immunol 2011;31:233-63.
- Orlikova B, Tasdemir D, Golais F, et al. Dietary chalcones with chemopreventive and chemotherapeutic potential. Genes Nutr 2011;6:125-47.