The onset of yolk-sac edema in Pacific herring (Clupea pallasi) due to environmental stressors present during an oil spill in an estuarine environment
2 EcoAnalysts Inc., Port Gamble, Washington, USA
3 Peapod Research Watermark Dr, Allendale, USA
Received: 13-Sep-2017 Accepted Date: Oct 28, 2017; Published: 10-Feb-2018
Citation: Word JQ, Word JD, Pinza MR, et al. The onset of yolk-sac edema in Pacific herring (Clupea pallasi) due to environmental stressors present during an oil spill in an estuarine environment. J Env Chem Toxicol.2018;2(1):1-11.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
OBJECTIVE: A natural resource damage assessment (NRDA) was conducted by federal and state trustees after the 2007 Cosco Busan oil spill (CBOS) in San Francisco Bay. Pacific herring embryos were collected from selected central bay sites and examined for early-life stage developmental injury; reported anomalies were thought to have derived from exposure to CBOS fuel oil. Chemical analyses of embryonic tissues did not demonstrate a clear pathway of CBOS exposure. Since the herring embryos were collected from shallow, dynamic estuarine waters, we conducted a controlled laboratory study to further investigate the possible effects on developing herring embryos caused by the fluctuating environmental stressors that naturally occurred in these shallow intertidal spawning areas (especially shifts in temperature, salinity, and ultraviolet light exposure that is intensified as a consequence of stranding in shallow water).
METHODS: This unique laboratory study evaluated effects on herring development caused by a suite of estuarine environ-mental stressors in the absence of CBOS by replicating near shore environmental conditions occurring at two sites within the central bay during the 2008 post-spill spawning. In addition to assessing developmental response endpoints such as body axis defects following a qualitative scoring process, we developed a new approach for assessment of pericardial and yolk-sac edema based on quantified measurements.
RESULTS: A significantly higher incidence and intensity of yolk-sac edema was observed in the embryos exposed to temperature and salinity changes, whereas pericardial edema did not occur in larval specimens at statistically significant levels compared to controls. Embryos exposed to ultraviolet light were associated with a higher incidence of body axis defects in larval specimens relative to the control. These experimental results indicate that fluctuating environmental conditions contributed to the abnormalities observed in naturally spawned herring collected during the post-spill spawning event.
Abbreviations
ANS Alaska north slope crude oil; ANOVA Analysis of variance; BD Body depth (vertical axis); BML Bodega marine laboratory; CBO Cosco busan fuel oil; CBOS Cosco busan oil spill; DPF Days post fertilization; DPH Days post hatch; ELS Early-life stage; NOAA National oceanic and atmospheric administration; NRDA Natural resource damage assessment; PAH Polycyclic aromatic hydrocarbons; PD Pericardial depth (vertical axis); PP Peninsula pt San Francisco; PSQ Pt san quentin San Francisco; SCAT Shoreline cleanup and assessment technique; SL Standard length; YD Yolk-sac depth (vertical axis); YL Yolksac length (horizontal axis); UV Ultraviolet irradiance
After the container ship Cosco Busan struck the Bay Bridge in San Francisco Bay in November 2007 and spilled 58,000 gallons of IFO 0380 fuel oil (US Department of Homeland Security, NOAA Incidentnews. gov), the oil was observed by Joint Shoreline Cleanup Assessment Teams (SCAT teams) in other areas of the bay historically used as spawning habitat by Pacific herring [1]. Larval Pacific herring (Clupea pallasi) were selected as a biological indicator species to determine potential impact to near shore fish populations created by exposure to Cosco Busan oil (CBO). Assessment of the potential impact to early life stage (ELS) Pacific herring (Clupea pallasii) caused by the Cosco Busan oil spill (CBOS) launched three separate investigations and provided an opportunity to examine a suite of biological response patterns occurring as a result of three scenarios:
• The CBOS spill event in estuarine conditions [investigation conducted in 2008] [1-3]
• Fuel oil exposure in mesocosm experiments [investigation conducted in 2009] [4-6]
• The present study, conducted in 2010 in the absence of CBO with fluctuating environmental stressors replicating the conditions recorded at two locations of field investigations after the spill [7].
Naturally spawned eggs were collected from the intertidal zone in February 2008 by the NRDA team at locations within the bay where CBO had been observed by the SCAT team as well as from potentially non-oiled locations. Eggs and hatched larvae were examined by members of the damage assessment team for multiple biological endpoints representative of the health and/or abnormalities in the development of early-life stages of herring. The team reported assessment of hatching success and developmental abnormalities indicating that several of the sites were severely impacted [1]. Many of the reported abnormalities were similar to anomalies associated with Alaska North Slope (ANS) syndrome (and blue sac disease) suggesting a potential cause and effect relationship between CBO and the observed anomalies [8- 12]. However, chemical analysis of eggs collected from the impacted areas failed to demonstrate the presence of the characteristic chemical signature indicative of exposure to CBO [1], therefore, a clear linkage of CBO exposure and the associated anomalies observed in the naturally spawned eggs collected in the 2008 post spill investigation could not be established.
In 2009, a series of mesocosm studies were conducted to document that CBO exposure would produce anomalies similar to those reported for the naturally spawned herring larvae collected in 2008. These experiments documented that morphological, physiological and developmental abnormalities occurred in the presence of CBO [13]. Chemical analyses of water and larval tissue samples documented exposure of embryos to CBO, uptake of PAHs into tissues, and that a unique chemical signature of CBO was present in the water and within the tissues [14]. While these experiments were severely compromised by numerous experimental artifacts such as gamete quality, extreme shifts in temperature, pH, algal fouling, and fluctuations in UV exposure, the chemical signature of exposure to CBO was present even at the lowest exposure concentration [14]. Because these mesocosm experiments were so severely compromised by extreme natural fluctuations, definitive determinations of cause and effect relationships for CBO exposure comparable to the field observations and herring development could not be established from the 2009 study. We hypothesized that fluctuating environmental stressors may have contributed to decreased herring hatching success and normal development during the field evaluations in 2008 and the mesocosm observations in 2009. The variable and fluctuating environmental conditions occurring at the intertidal spawning locations in 2008 may have been a contributing feature of the nearshore environment causing reduced hatch rates and compromised early development and physiological health of larvae.
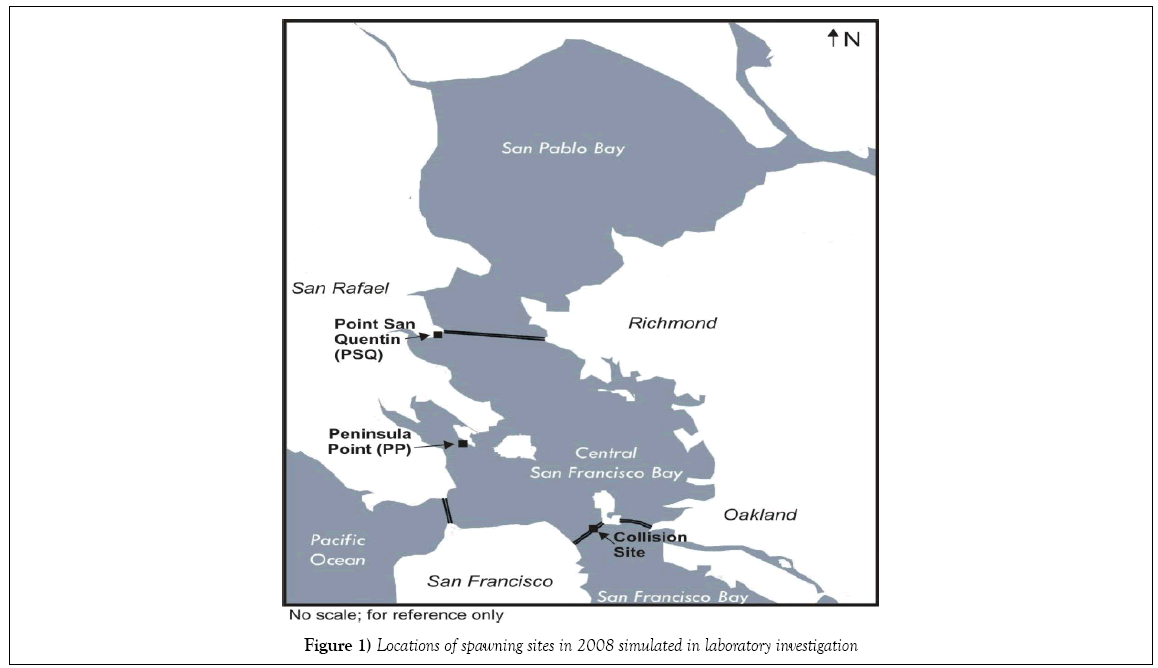
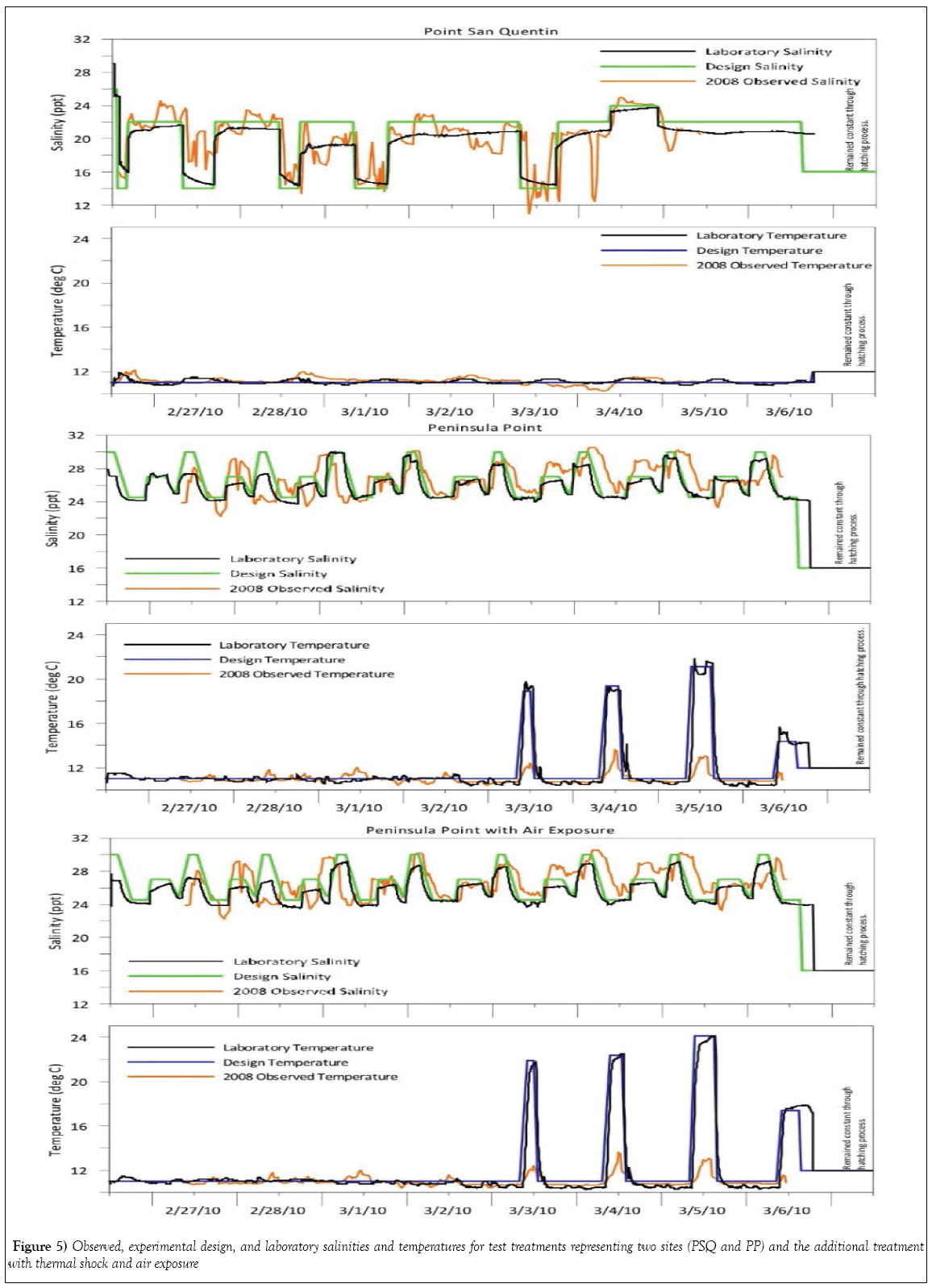
The present study was undertaken to explore the influence of fluctuating environmental stressors on developing herring embryos and post-hatch larvae in the absence of oil. Common metrics of growth (standard length, and hatching success), morphological development (body axis deformities) and physiological functioning (particularly the onset of pericardial and/ or yolk-sac edema) were examined as endpoints. Natural environmental fluctuations were recorded in oceanographic data collected from near shore locations within the central portion of San Francisco Bay, and upon detailed examination, we observed that the same oscillations were not evident at all sites. Two of six locations investigated during the post-CBOS resource damage assessment were selected for further study under laboratory conditions mirroring those recorded in the field: Peninsula Point (PP) represented an area potentially impacted by the CBOS, while Point San Quentin (PSQ) represented an area to the north furthest away from the CBOS and was designated as a reference site for the NRDA investigation (Figure 1). Although the two field locations selected for the present laboratory study are situated within the central bay region of San Francisco Bay, different salinity and temperature profiles were recorded at PP compared with PSQ during the collections by NRDA team members of naturally spawned herring embryos in 2008. The PSQ site salinity records demonstrate a large variation in salinity profiles responding to the relative dominance of riverine influence from the Sacramento River or oceanic influence from the Pacific Ocean. Collections at PSQ were made during a sequence of high tides, which effectively drives more saltwater in from the Pacific Ocean into the central bay. In contrast, low tides and unseasonably warm temperatures were noted to occur during daylight hours during the post-spill spawning events in the vicinity of Peninsula Pt. during the collections, which would have exposed incubating embryos to shallow water stranding and increased thermal and UV exposure. Previous studies have been performed to determine the effects of different temperatures and salinities on developing embryos [15-18]; however, experimental treatments in these studies were performed at static temperatures and salinities. To our knowledge a laboratory study designed to simulate real-time changing estuarine conditions and consequent onset of developmental anomalies such as pericardial and yolk-sac edema had not been conducted.
Material and Methods
Field collected environmental data from Peninsula Pt. and Pt. San Quentin
This laboratory study was designed to demonstrate the extent to which fluctuating environmental conditions such as temperature, salinity, increased UV intensity, and transient air exposure occurring in shallow intertidal waters may contribute to abnormal embryonic development of Pacific herring. Data for salinity and temperature cycles were collected from continuously recording water quality instruments placed in the two study locations. The site records were reviewed and laboratory seawater exposure regimes were adjusted to replicate these specific parameters during an 8 day continuous flow experiment with different exposure scenarios.
Production of live embryos from field-collected herring
Eggs from mature fish captured in Richardson Bay were artificially spawned and fertilized in the laboratory. Spawning adult male and female Pacific herring were collected between February 19 and 24, 2010 from shallow Richardson Bay spawning grounds (centered at 37.87°N latitude, 122.48°W longitude) in the San Francisco estuary. Fish were captured using fishing rods rigged with Sabiki lures; gonads were removed immediately on board the vessel and placed into individual glass Petri dishes, sealed with parafilm and tape, labeled and placed over paper-covered ice in coolers for transport to the laboratory. Upon arrival at the laboratory, the gonads were composited from adult male and female fish. Initially, the study design included parallel testing of two age classes of gravid female herring. However, eggs collected from older, larger females (3 year and older; ≥ 180 mm standard length) were not of sufficient quality and did not achieve minimal control hatch rates to ensure a successful test. Therefore, the data brought forward represents results from embryos from females in the younger age class (2 year old fish; 160 mm standard length).
The fish gametes were artificially spawned and fertilized within 4 days of collection in San Francisco Bay following methods outlined in Dinnel et al. [19]. Test water was prepared with 0.45 μm filtered seawater from Hood Canal Bay, Washington diluted with deionized water to create fertilization water at test conditions of 12°C and 16, 22 and 28 ppt salinity. Testes were chosen based on size; larger gonads were considered more mature and viable. Eggs were fertilized following protocols in Dinnel et al. [19] and extruded onto glass microscope slides (25 mm × 75 mm). Approximately 100 eggs were distributed onto each slide and the slides were placed in glass dishes containing 500 ml of sperm solution. After a 30 min exposure, slides were assessed for relative percent fertilization and then removed, rinsed gently with control seawater at the appropriate test salinity, and placed in separate incubation chambers within individual 10 L aquaria.
Test treatments
Table 1 summarizes test conditions for each treatment. Two control treatments (Control, Control-2) were run under optimal conditions for herring embryos from San Francisco Bay, i.e. temperature of 12 ± 1°C and salinity of 16 ± 1 ppt [18,20] with different levels of UV irradiance. The test treatments required temperature and salinity to fluctuate outside of optimal quality control ranges in order to simulate those conditions experienced by developing eggs after the oil spill. One test treatment (PSQ) simulated the fluctuations in salinity that had occurred at Point San Quentin during the time period of egg collections by the NRDA team. Three test treatments (PP- 1, PP-2 and PP-3) approximated the complexity of variations in environmental conditions at Peninsula Point (i.e., water temperature, salinities and tidal height fluctuations). The first two treatments examined embryo exposures that may have occurred while submerged within the water column: PP-1 tested variable salinity and temperature exposure from point of fertilization through embryonic development as if embryos were submerged in the water column, while the PP-2 treatment examined the effect of fluctuating temperatures only. Variations in exposure conditions for PP-3 incorporated periods of shallow water stranding, which was expected to induce thermal shock and possible aerial exposure based on recorded extreme tidal cycles and coincident unseasonably warm air temperatures during the collection of eggs by the NRDA team from the PP site (NOAA weather service).
| Treatment | Primary variables | Fertilization salinity (‰) |
Salinity regime (Target ±1%) |
Temperature regime (Target ± 1°C) |
UV (µW/cm2) |
Aerial stranding | Water quality | |
|---|---|---|---|---|---|---|---|---|
| Dissolved oxygen (mg/L) |
pH | |||||||
| Control | Optimum, low UV | 16 | 16 | 12 | <5 | >4.8 | Ambient (7.7) ± 0.5 | |
| Control-2 | Optimum, moderate UV | 16 | 16 | 12 | 115-135 | |||
| PSQ | Salinity | 22 | 14-24 | 11 | 115-135 | |||
| PP-1 | Salinity*, temperature | 16 | 24.5-28 | 11, periodic increases to 22 | 115-135 | |||
| PP-2 | temperature | 28 | 24.5-28 | 11, periodic increases to 22 | 115-135 | |||
| PP-3 | Thermal shock, aerial exposure | 28 | 24.5-28 | 11, periodic increases to 24 | 115-135 | Yes | ||
*Change in salinity from spawning/fertilization to treatment
Table 1: Experimental test conditions
Experimental array
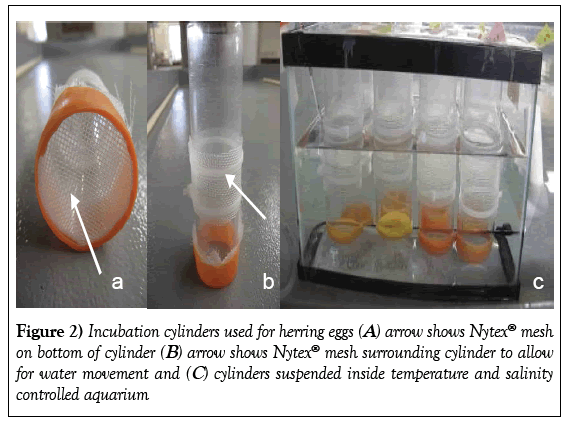
Four replicate glass slides containing the fertilized eggs were placed within individual incubation chambers suspended from a Plexiglas frame into a 10 L glass aquarium. Three replicates were used for experimental results; a fourth replicate was used for daily observations to minimize laboratory impacts on the test replicates. The incubation chambers were created from polycarbonate cylinders fitted with a 1 mm Nytex mesh screen covering the bottom end of each chamber with an additional opening in the center which was also covered with a secured Nytex sleeve. The Nytex screens provided a conduit for water exchange within each incubation chamber. The chambers were suspended above the bottom surface of the aquarium at a depth which provided 150 ml (85 mm depth) of water volume for each chamber (Figure 2). The 24 samples (6 treatments × 4 replicate slides suspended in each aquaria) were identified as sample numbers 1 to 24 with only enough information about the treatment regime provided to allow accurate testing conditions.
Test aquaria received a constant renewal of flowing source water maintaining a volume of 6 L. Seawater was prepared in a 20 L glass carboy; 0.45 μm filtered seawater was diluted with deionized water to the appropriate test salinity. Water was delivered directly to the aquaria from the carboys using peristaltic pumps. Test water flowed into the aquaria via Tygon tubing connected to the carboys and exited the aquaria through an adjustable port located on the opposite side of the tank. Aquaria and carboy reservoirs were placed in water-bath tables with flowing seawater. Temperature control was automated by Fuji Electric PXR4 Micro-controllers) to maintain to control test temperatures (12 ± 1°C) whereas water-bath temperatures for the test treatments were individually modified to mirror onsite buoy data recorded for the post-spill period. The controller was programmed to change temperatures according to the testing plan. Trickle-flow aeration (roughly two bubbles per second) was provided to each tank; temperature and salinity were monitored continuously throughout the test with recording YSI Professional Plus water quality meters. Dissolved oxygen, pH, temperature, and salinity were measured in each aquarium daily using Orion 5 Star meters. Salinities fluctuated according to a pre-determined schedule by altering the incoming water supply to freshly prepared water of the desired temperature and salinity, and adjusting the flow rate of the pump to supply the water flow over the scheduled time period. When no salinity changes were scheduled for a given treatment, and for the control aquaria, water flow was set such that a complete renewal of water occurred every 24 h. (One test treatment, PP-3, received increased thermal and aerial exposure to simulate exposure during a low-tide cycle. These conditions were achieved by moving the test array containing the PP-3 embryos to a separate aquarium without seawater and placing it inside a temperature-controlled incubator equipped with UV light for 3 periods of ~1 h duration, simulating the expected effects of the low tide cycle noted in records for the PP site. Dissolved oxygen and pH were measured daily and remained within target limits throughout the test period in all samples (Table 1). Temperature and salinity in the control and CUV treatments remained constant throughout the test as noted by a log created by continuously recording salinity and temperature measurements collected by a monitoring device positioned in the water bath where both treatments were located.
The experimental photoperiod mirrored the hours of daylight during February 2008 (NOAA, Greg Baker pers. comm); treatments received 13 h light and 11 h darkness daily matching conditions in San Francisco Bay during the 2008 post-spill spawning period. Lighting was provided by fluorescent light ballast containing one fluorescent bulb (Duro-Test Vita-Lite; 40W, 5500°K, 91 CRI) and one standard fluorescent bulb (Phillips F40CW) placed within 12” above the incubation chambers. All incubation chambers were left uncovered to prevent UV loss. Light intensity was measured at the water level of test chambers with a Reed LM-811X meter. The control treatment was exposed a low level of UV from the standard fluorescent light bulb (measured as <5 μW/cm2) only. All other test treatments including the Control-2 treatment were exposed to higher UV irradiance, ranging from 115 to 135 μW/cm2.
Determination of fertilization success at 48 h
Fertilization success was assessed at 48 h post fertilization; the results are shown in Table 2. Most of the embryos were in transition from blastula stage to epiboly as described by Hill and Johnston [21]. The fertilization rates ranged from 80.5% to 97.3%. Percentage fertilization was calculated by subtracting the unfertilized and unhardened eggs from the total eggs counted. At 48 h, the percentage of fertilized and viable embryos ranged from 43 to 80.3%. The control treatment at 48 h had 70.3% fertilized and viable embryos validating the test [22].
| Treatment | Fertilized (% Total Eggs) |
Unfertilized or unhardened (% Total Eggs) | Dead embryos at 48 h (% Fertilized Eggs) |
Viable fertilized embryos (% fertilized Eggs) |
|---|---|---|---|---|
| Control | 81 | 19 | 30 | 70 |
| Control-2 | 86 | 14 | 30 | 70 |
| PSQ | 86 | 15 | 20 | 80 |
| PP-1 | 97 | 3 | 57 | 43 |
| PP-2 | 81 | 20 | 27 | 73 |
| PP-3 | 97 | 3 | 31 | 69 |
Table 2: Fertilization rates determined at 48 h post fertilization
Slides were carefully removed from the test chambers and placed into glass crystallization dishes filled with water at appropriate test conditions. Each slide was photographed with a Canon G10 Power Shot digital camera mounted on a tripod to provide a stable and consistent platform and then the slides were viewed using a Nikon dissecting microscope. Eggs on each slide were enumerated and classified as fertilized, unfertilized, or dead; the slide was placed over a grid matrix to facilitate accurate counts. Eggs were considered fertilized and viable if there was a membrane surrounding the egg indicating water uptake post-fertilization had occurred. Unfertilized eggs were those with no apparent yolk membrane. Unfertilized and dead eggs, as well as densely clustered eggs, were removed from the slides with forceps without damaging developing eggs in order to retain 80 to 100 eggs on each slide. Care was taken to minimize the amount of time the slides were in crystallization dishes to prevent temperatures in the dishes from rising above test conditions.
Assessment of hatch success at 8 dpf
The fertilized eggs were incubated in the flow-through test aquaria at test exposures for 8 days after which time all slides were transferred to individual 100 × 50 mm crystallization dishes maintained at a constant temperature of 12°C and salinity of 16 ppt water; the dishes were used to secure the emerging hatchlings from escape or entrapment on the mesh screen that was used as part of the flow-through incubation chamber. Water was renewed in the dishes daily until hatch completion. At 8 days post fertilization (dpf) the 24 slides were photographed again with a Canon G10 Power Shot digital camera. Eggs were classified and enumerated as unfertilized, live normal, live abnormal or dead. Live eggs were considered normal if they had no apparent opacity and had two distinct eyes. Abnormal eggs were embryos with a viable heartbeat but with noticeable abnormalities or distorted physical features.
Some abnormal eggs were underdeveloped or opaque; partially hatched larvae were also included in this group. Dead embryos included those with no beating heart or a lack of movement. Dead eggs were grouped into two categories; those that had achieved later-stage development indicated by the presence of eyes were categorized as “dead (eyed).” Those with no visible eyes, suggesting early mortality, were counted as “dead (other).” After slides were digitally recorded, those with unhatched eggs were placed back into the crystallization dishes containing water at 12 ± 1°C and 16 ± 1 ppt salinity; forceps were used to transfer each slide into a clean pre- labeled crystallization dish containing the renewal treatment water (16 ±1 ppt and 12 ± 1°C). When hatch was complete (no viable eggs remaining on the slide), final photographs of slides were taken using the Canon Power Shot camera.
Test termination and preparation of larvae for image processing
Hatched larvae were retained in respective crystallization dishes until processing. Within 12-24 h of hatch, herring larvae were briefly anesthetized for viewing purposes (typically for 5 min) using a low dosage of tricaine methanesulfonate (MS-222; 50 mg/L). The 50 mg/L MS-222 concentration was selected as optimum based on anesthetic trials conducted using a series of concentrations ranging from 25 to 200 mg/L MS-222. This concentration was the lowest dosage which immobilized the larvae for imagery and showed no visible adverse effects to the larvae. Higher concentrations of MS-222 showed signs of edema. No fixatives or preservatives were used prior to assessment of normal development, since the associated chemicals can compromise the tissue and skeletal integrity of the specimen. Larvae were not anesthetized for an extended period of time (~5 min) prior to obtaining digital image records. Each specimen was viewed within a crystalline dish using a high-powered microscope (Olympus SZX7 with a DP72 high performance Peltier cooled stage) integrated with a 12.8 MP digital color camera with digital recording software to record each specimen in a lateral plane for archived digital records. Whole-body photo records for each specimen were captured at 25x magnification, and close up views of the peritoneal area were captured at 56x; subsequent image analysis using Image J software magnified each digital image up to 400% so that the specific yolk and pericardial membranes could be closely examined. Once newly hatched larvae were imaged, they were humanly euthanized using a lethal dose of MS-222 (50 mg/L) according to laboratory protocols and then preserved in Davidson’s solution for archival purposes.
Measurement of endpoints
Quantitative measurements of standard length and pericardial and yolksac attributes of each larva were made using analytical tools for lineal measurements available in Image J software (http://rsbweb.nih.gov/ij; units were in pixels). The measurements performed on each larva--standard length, length and depth of the yolk-sac and depth of pericardial region--are shown in Figure 3. Herring larvae are mostly transparent, making the distinction between pericardium and yolk sac difficult at times. Additionally, the posthatch development of herring larvae proceeds extremely rapidly, and the changing relationships of these cavities make it imperative to properly identify the pericardial boundary prior to assessment of edema. A membrane, the transverse septum, defines the posterior end of the pericardium, securing cardinal and hepatic veins and stabilizing future organ growth regions (e.g. liver) as illustrated in Figure 3. Recognizing the functional aspect of the transverse septum is an important refinement to methods used previously to describe pericardial edema [23-25]. Identification of the septum is a crucial step in accurately delineating the pericardial and yolk-sac regions and making proper assessment of the respective sites of edema. While the pericardial and yolk cavities are adjoined in the early stages of larval development, after hatch the pericardial and yolk-sac regions of the larvae become spatially separated by the developing organs in the pectoral fin girdle. Further illustrations of allometric changes in the pericardial and yolk sac and colocating the transverse septum during these accelerated development stages are presented in the Supplemental Figures 1 and 2. While the yolk sac is ultimately resorbed into the body cavity approximately 4 to 6 days post hatch (dph), the location of the transverse septum remains constant relative to the anterior edge of the dorsal fin where it joins the body wall and appears to be co-located with the anterior edge of the base of the pectoral fin. These two visual reference points were helpful in ascertaining the position of the septum in images where it is not as clearly visible. In these cases, it was also helpful to alternate between light and dark fields, a tool available with Image J software.
The measurement of the depth of the pericardium (PD) and total body depth (BD) were made at the location of the transverse septum; the ratio of these two measurements (PD: BD) is indicative of the relative amount of distention of the pericardium and is unit less. The ratio of the length of the yolk sac to the depth of the yolk at midpoint demonstrates a relative enlargement of the yolk-sac area when it occurs; this ratio is also unit less. The use of the ratios rather than dimensional measurements allows for comparisons among datasets having different magnifications of the images or measurement units (pixels or millimeters).
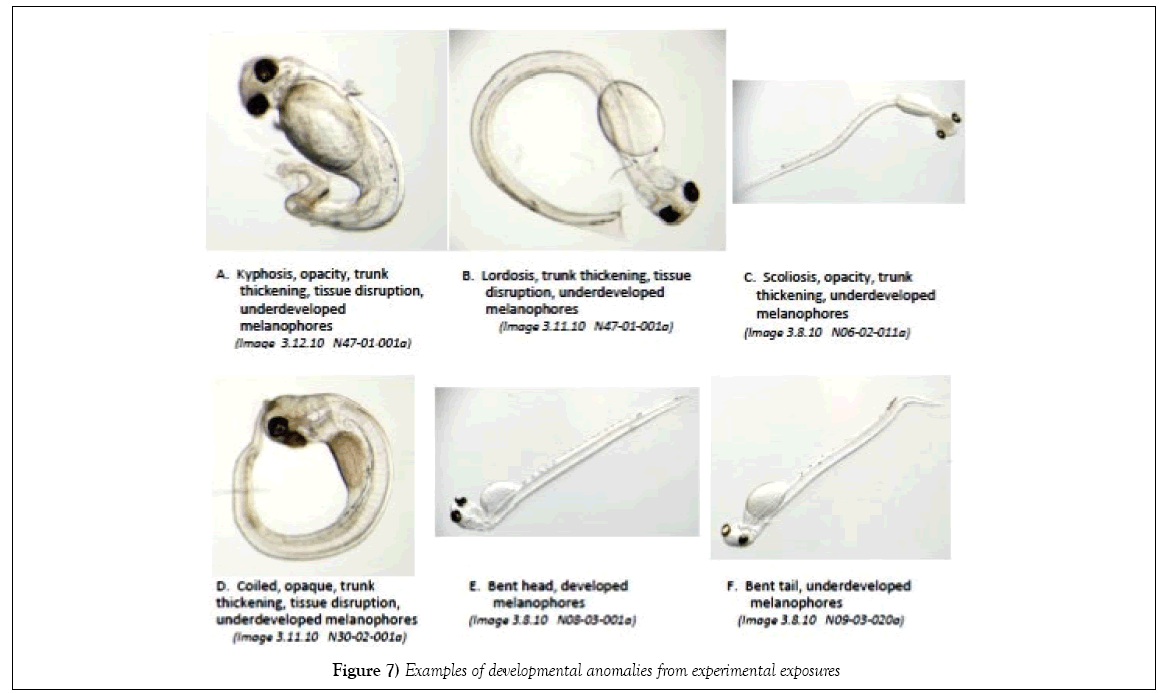
For each larva, body axis defects were scored semi-quantitatively; the deviation of the body lateral line from the horizontal plane were assigned an indexed degree of anomaly ranging from 0 (no deformity) to 3 (severe deformity), following the approach presented in McIntosh et al. [24]. The associated type of dysfunction were noted: lordosis (convex arched spinal curvature), kyphosis (concave arched spinal curvature), scoliosis (sideways spinal curvature), stunted and thickened trunk (shortened trunk, thick body mass) and craniofacial (jaw) abnormalities.
Data analyses
All statistical analyses of hatch success and quantitative and semi-qualitative endpoints were performed on the mean of the measurement or score for all larvae in each replicate sample. Frequency of occurrence of edema and body axis defects was calculated by the number of larvae in each replicate exhibiting the abnormality divided by the total hatched larvae in the sample. Larvae with multiple defects were scored in this way for each of the defect types. Treatments were compared with a one-way analysis of variance (ANOVA) with significance set at 0.05. All treatments were then compared to the control sample using a Tukey’s HSD statistical test [25], a one-tailed test was used for scores and frequencies and two-tailed tests were used for measurements. All statistical tests were performed with SAS/STAT® software [26].
During the imaging of live specimens, many larvae were observed to be in a later stage of yolk-sac absorption (Figure 4). The larvae in the late stage yolk-sac absorption stage were encountered on days 10 and 12 post-fertilization when the majority of hatch occurred. Because of the large number of larva at hatch there was an increase in the time required for processing the specimens. The numbers of larvae found in this more advanced stage are attributed to their continued development between hatching and the time at which processing actually occurs. On the high hatch dates 9 to 11 days after fertilization, up to 20 randomly selected larvae were imaged from each sample. When more than 20 larvae hatched from a sample in a day, the remaining larvae were photographed after the entire set of laboratory samples was processed. While the overall percentage of larvae in the late-stage category was relatively small (7%), they were not evenly dispersed across the treatments; those treatments observed later in the day had more time for the hatched larvae to develop before imaging occurred. Consequently, the larvae with visually evident resorption of the yolk sac were excluded from assessment of yolk sac and pericardial edema to avoid biasing the results.
Results
Attainment of test experimental conditions
Salinity and temperature were intentionally fluctuated for three test treatments representing a potentially oiled site with multiple stressors, Peninsula Point (PP-1, PP-2 and PP-3), while only salinity was varied in the treatment representing Point San Quentin (PSQ). Changes in laboratory temperature and salinity regimes simulating conditions naturally occurring during February 2008 were successfully achieved as shown in Figure 5. The targeted thermal spikes in all PP treatments were intentional; differences in targeted laboratory temperature exposure and field recordings for PP is due to a perceived margin of temperature elevation not accounted for by the position of the field recordings made by the loggers at deeper water depths, which did not address more extreme temperature shifts and potential for aerial exposure in shallower intertidal water depths resulting from low-tide cycles. Exposure to air in the thermal shock treatment was maintained for a briefer length of time than likely occurred during these low-tide cycles in PP during the 2008 collections.
Pre-hatch development and hatch success
Determination of early death of embryos was based on opacity of the embryo or a disrupted white tissue mass within the chorion often associated with arrested development during the blastula stage (Table 2). Only one treatment (PP-1) simulating fluctuating salinity conditions from fertilization through the remaining developmental states plus temperature variation had greater than 50% dead embryos at 48 h; the remaining treatments showed 30% or fewer dead embryos. The percentage of viable eggs at a later stage in development was calculated by dividing the viable eyed eggs by the number of fertilized eggs. Results were different among treatments: PP-1 had the lowest percent viable embryos (43%) while PSQ had the highest percent (80%), the remaining treatments fell within this range (Table 2).
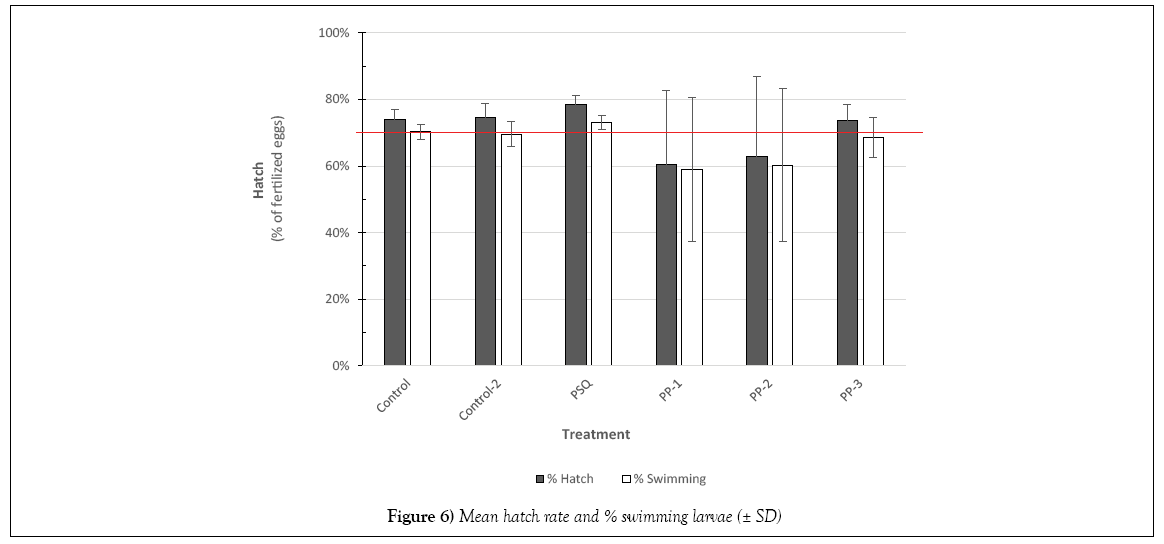
Over 600 larvae hatched during the period from 9 to 14 dpf. Larvae observed swimming in the incubation chambers were tallied in addition to the total number hatched from the glass slides into the glass dishes. Hatch rates were determined by the number of fertilized eggs remaining on the slide at 8 dpf. As shown in Figure 6, PP-16 and PP-28 treatments showed the most variability in hatch rates. In addition, in the control samples the viable embryos at 2 dpf (70%; Table 2) were essentially the same as the swimming hatchlings (70%) indicating, in general, those embryos surviving past 2 dpf in control treatments preceded to normal hatch.
Natural population variability was observed in the control treatments as evidenced by the number of abnormal larvae (2 to 5%), similar to incidence of abnormal development cited for control samples in other studies with San Francisco Bay herring [1,4,27,28].
Growth, morphological and physiological endpoints
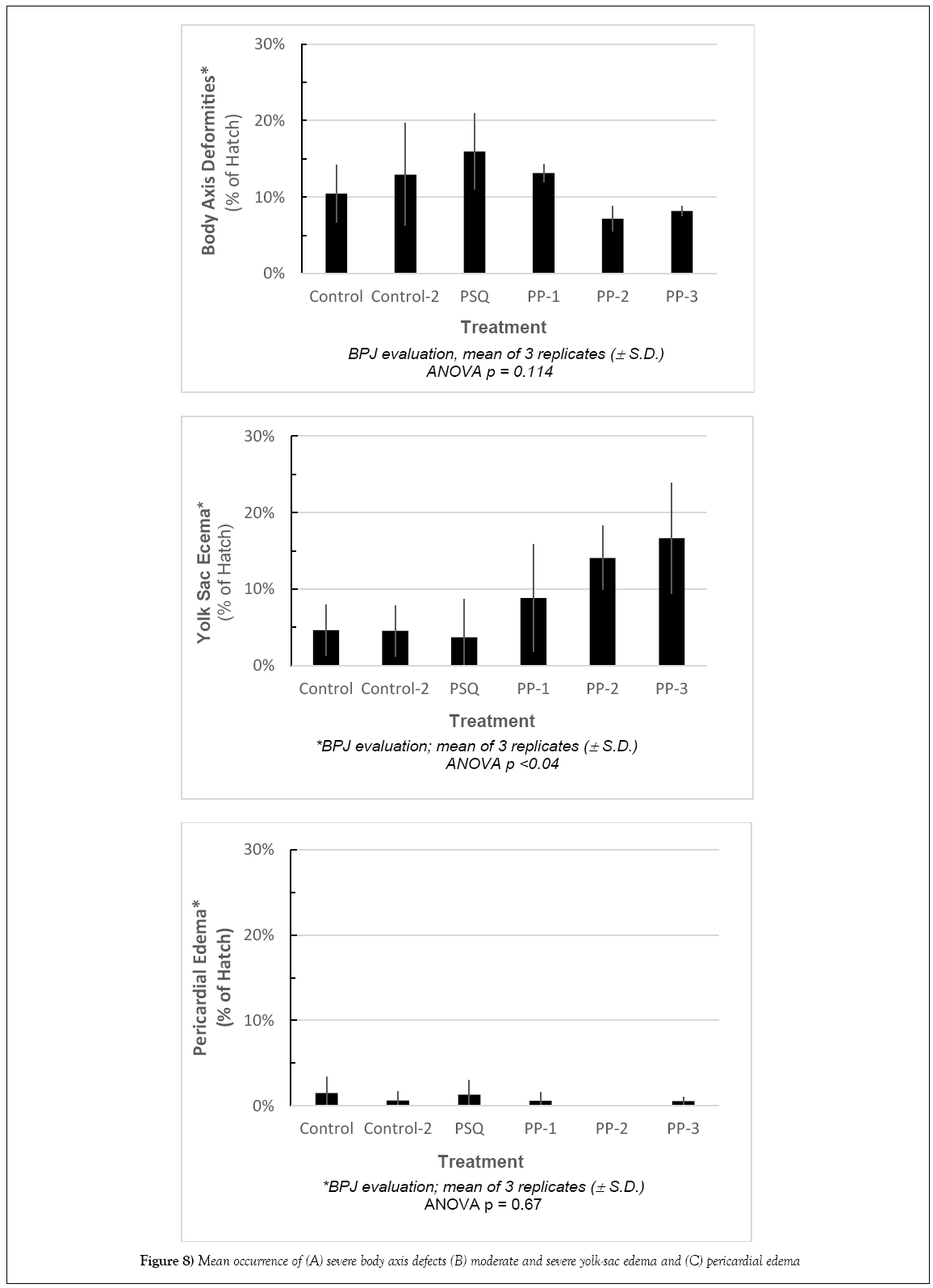
In comparison to the control, there were no significant differences in Standard Length (SL). The shortest lengths were found in the temperaturestressed development (PP-3) while the largest were in treatments PP-28 and PSQ. The frequency of body axis defects was calculated based on semiqualitative assessment. There were no significant differences among stations compared with the controls (Figures 7 and 8).
The ability to quantitatively measure deviations from normally developing larval fish in assessment of pericardial and yolk-sac edema has important implications for establishing cause and effect relationships producing the specific edema syndromes. Baseline ranges for normal metrics are represented by over 90 control measurements. Ratios of relevant allometric yolk and pericardial measurements were computed and tested for differences from control treatments with ANOVA and Turkey’s HSD statistical tests. Results of ANOVA tests on the quantitative measurements and examination of the PD: BD ratio summary data indicated that occurrences of pericardial edema were significantly different. Turkey’s HSD evaluation revealed no significant differences in PD from control, and only PSQ-22 showed a significant difference from control for BD. The ratio of BD: PD showed no significant differences from control, (Table 3). ANOVA revealed significant differences among treatments for YD, YL, and YD: YL. The differences from the control treatments include PP-28 and PSQ-22 for YL; PSQ-22, PP-TS, PP-16 and PP-28 for YD, and CUV, PP-28, PP-TS and PP-16 for the ratio YD: YL. Treatments simulating the environmental stressor sites (PP-28, CUV, PP- 16 and PP-TS) were found to have significantly higher occurrences of yolk-sac edema as defined by these measurements (Table 3).
| Measurement | P | MSDa | Control | Control-2 | PSQ | PP-1 | PP-2 | PP-3 |
|---|---|---|---|---|---|---|---|---|
| Standard length (mm) | <0.001 | 1.20 | 8.35 | 8.43 | 8.48 | 8.35 | 8.48 | 8.22 |
| Pericardial depth (PD: Notochord to ventral edge, mm) | <0.001 | 0.03 | 0.27 | 0.28 | 0.24 | 0.29 | 0.26 | 0.29 |
| Body depth (BD: Dorsal to ventral edge, mm) | <0.001 | 0.04 | 0.50 | 0.52 | 0.47 | 0.51 | 0.48 | 0.52 |
| Ratio PD:BD | <0.001 | 0.02 | 0.53 | 0.54 | 0.51 | 0.55 | 0.54 | 0.55 |
| YD (Yolk depth, mm) | <0.001 | 0.11 | 0.52 | 0.53 | 0.46 | 0.53 | 0.46 | 0.51 |
| YL (Yolk length, mm) | <0.001 | 0.31 | 0.96 | 0.94 | 0.89 | 0.87 | 0.80 | 0.88 |
| Ratio YD:YL | <0.001 | 0.11 | 0.55 | 0.56 | 0.52 | 0.61 | 0.58 | 0.59 |
MSDa Minimum significant difference
Table 3: Results of ANOVA and Turkey test results from quantitative larval measurements, values significantly different from control are in bold. Refer to Figure 3 for description of measurements
Discussion
The use of larval herring has an established precedent in evaluations of petroleum impact; published literature has been augmented by resource damage assessments using the Exxon Valdez oil spill (EVOS) in 1989 [9,23,24,29-35]. A standardized bioassay method for examination of earlylife- stage herring recently published includes a similar suite of biological endpoints such as hatch success, growth, pericardial irregularities (heart rate), and presence/absence of yolk-sac edema [21]. The present study evaluated similar endpoints with laboratory exposures based on nonchemical environmental stressors.
Environmental stressors examined during an 8 days laboratory experiment simulating conditions present at Peninsula Point and Point San Quentin during the herring spawn following the CBOS were: fluctuating salinity and temperatures, exposure to UV light, and the influence of air exposure during intertidal stranding episodes during embryonic development. A comparison of targeted salinity and temperature regimes versus measured salinity and temperature data collected during the experiment indicated that we successfully matched the salinity and temperature profile records for the two sites, and achieved a sequencing of elevated water temperature and exposure to warm air temperatures to reflect the more extreme conditions which likely prevail during low tide events. The air exposure in the PP-3 treatment was limited to 1 h sessions in a temperature-controlled incubator in the laboratory to incorporate a representation of slack tidal periods which is likely less than the embryos would have experienced in 2008; consequently, the experimental exposure likely underrepresents the potential stress that may have occurred in 2008 in the shallow intertidal habitat. Test results were based on similar developmental stages of post-hatch larvae. For herring, embryonic development occurs rapidly during organogenesis and larval development is accelerated over the first few days post-hatch [21]. This rapid growth results in a narrow time window for making comparable developmental assessments; allowing variability in developmental age severely handicaps the quality of data assessment. Dechorionation disrupts the very rapid growth that occurs during organogenesis, and it is more difficult for the investigator to accurately determine the boundary between the pericardium and yolk sac, especially in very early developmental stages (o and p). Additionally, the transverse septum has a curvilinear aspect during embryonic development that differs from the perpendicular orientation relative to the lateral body plane in naturally hatched larvae (Image 2B, SI). Therefore, our examinations of developmental and physiological anomalies were conducted on naturally hatched larvae to eliminate potential bias that may be induced by using DE chlorinated larvae. Previously, assessment of edema has been problematic because transparency of herring larva makes distinctions between membranes and cavities, such as the peritoneal and pericardial cavities, difficult to determine. Recent advancements in microscopy and digital imagery enable precise delineation of pericardial and yolk areas, if the examiner identifies the transverse septum which clearly separates the pericardium from the peritoneal cavity. The presence of body axis defects is visually apparent and becoming standardized in reporting. A refined approach using a severity index to assign relative degree of observed biological stress has been employed [24], but this approach is also semiqualitative. Edema has been reported in the scientific community as a significant biomarker of PAH exposure, however previous evaluations have largely been based on subjective and qualitative observations that reflect the experience of the investigator (i.e., best professional judgment). Incardona et al. [36] conducted laboratory studies with marine and freshwater fish that examined effects of Alaska North Slope crude oil on embryogenesis, focusing on edema and cardiac dysfunction and the correlation of toxicity responses to tricyclic (three-ringed PAH) compounds [4,36,37]. Although there is mounting evidence of specific etiology of stress created by petroleum hydrocarbons, the limitations of qualitative evaluations compromise verification of these important biological assessments [38]. The incidence of abnormally developed embryos at hatch from the control treatment of the present study (10%) was within the range reported from other investigations (≤ 10%) [23,33]. Photographs of typical examples of abnormal development taken during the experiment are shown in Figure 7. Adverse effects were identified in association with the environmental factors that were examined (UV exposure, and the combination of temperature and salinity changes). A summary of semi-quantitative evaluations of body axis deformities, yolk sac edema, and pericardial edema is shown in Figure 8. Occurrences of developmental abnormalities varied in the UV treatments and appear to have increased in response to the added stressor of fluctuating salinity (PSQ). Three treatments were higher than the control treatment (Control-2, PP-1 and PSQ) whereas two treatments had lower incidence (PP-2 and PP-3). The trend of developmental skeletal abnormalities keyed to light conditions is supported by observations reported in Strähle et al. [39] in their study on zebra fish (Danio rerio) where exposure to UV in the absence of other contaminants caused injury during several phases of embryogenesis. In one example offered by Strähle et al. [39], separation of the blastoderm from the yolk cell was noted which formed a vesicle resting on the disintegrating yolk mass. Further UV exposure may disrupt the epiboly processes, which may lead to grossly retarded embryo development particularly in the trunk and tail regions. More recently, Dinnel et al. [19] evaluated the effects of the visible light spectra on embryo survival and normal hatch rate and concluded that embryo normal hatch rates were significantly reduced under shaded (170 lux) and strong-lighting (2650 lux), reducing normal hatch rates to 37% and 44% respectively, compared to 71% normal in dark (0 lux) conditions. Although these authors found that normal hatch rates were not affected by a range of salinity test conditions from 8% to 24%, these test results do not reflect the potential for the incidence of developmental anomalies to be magnified by the additive effect of multiple-stressor exposures.
Fluctuating temperatures and salinities were strongly associated with significant yolk-sac edema, both in intensity and incidence of the response. The treatment representing salinity only fluctuations (PSQ) showed occurrence of yolk-sac edema in 4% of the larvae, similar to the Control (5%) or the Control-2 treatment (5%) but well below the occurrence in the treatments representing multiple stressors under simulated Peninsula Point conditions. Yolk-sac edema was identified in 9 to 14% of hatched larvae in two treatments (PP-1 and PP-2) exposed to both temperature and salinity changes and in 17% of larvae that were additionally subjected to thermal shock simulating the low tide cycles (PP-3). Therefore it appears that the incidence of yolk-sac edema was more closely related to the temperature changes, air exposure, and possibly salinities between 24 to 28 ppt than to the changes in salinity in the lower range of 14-24 ppt with constant temperature. Research conducted by others indicates that physical stressors (temperature, salinity, pH, UV, dissolved oxygen in the absence of chemical contaminants) can create biological responses consistent with edema [15- 19,35]. The occurrence of yolk-sac edema associated with the non-chemical environmental stressors in the present study was comparable to reported incidence and intensity of edema at the sites potentially impacted by the oil spill in 2007 and investigated during the NRDA assessment in 2008 (NOAA/BML 2008).
In contrast to the elevated incidence of yolk-sac edema resulting from exposure to non-chemical environmental stressors, there was no relationship between any combination of non-contaminant environmental stressors and the onset of pericardial edema. All specimens exhibited a low incidence of pericardial edema when exposed to non-chemical environmental stressors, ranging from 0% to 1% (Figure 8C).
Conclusion
Peninsula Point was considered by the NRDA team to be an oiled site, and this location was reported to have large numbers of abnormal larvae [4]. As demonstrated by our test scenarios that closely mirrored field conditions at PP and PSQ, PP experienced larger fluctuations of temperature than PSQ. While the SCAT teams qualitatively documented the presence of CBO in the water column and at shoreline sites in 2007 [40], the herring eggs that were spawned in 2008 did not have a CBO chemical signature [1]. Incardona and Vines [4] demonstrated that herring embryos exposed to CBO using flow through mesocosm exposures resulted in PAH uptake into egg tissue that matched the chemical signature of CBO obtained from analysis of waterborne profiles. The diagnostic chemical signature of CBO in egg tissues from mesocosm studies and the dose responsive incidence of pericardial edema to elevated concentrations of CBO support the conclusion that CBO exposure can result in the manifestation of pericardial edema [14].
The absence of a CBO chemical signature in the tissues of the naturally spawned 2008 herring eggs indicates that these organisms were not likely to have been exposed to CBO. Additionally, our review of images of larvae from eggs collected during February 2008 provided by the damage assessment team using the same quantitative assessment approach presented in the present study indicated that similar rates of yolk-sac and pericardial edema were found in the present laboratory study and the 2008 field investigation. We conclude from the present study that the occurrence of multiple environmental stressors including rapidly changing temperatures and salinity plus thermal shock and air exposure can induce yolk-sac edema in Pacific herring from San Francisco Bay and the frequency of occurrence was similar to assessment of field collected embryos reported elsewhere [3,5,11,17,39-47]. These studies highlight the complexity of establishing biological response patterns associated with a particular contaminant or environmental condition. McDonald et al. [38] recommended that a quantitative or semi-quantitative method be developed to improve the reproducibility of experimental results among various investigators. McIntosh et al. [24] developed a semi-quantitative scoring method based on a severity index, but this is also based on subjective interpretations made by individual researchers. Although pericardial and yolk-sac edema have been widely used to represent sub lethal physiological markers of biological stress caused by petroleum and other contaminants, previous assessments of edema were based on qualitative or semi-qualitative assessments. The degree of variation associated with qualitative assessments can be significant and has led to conflicting conclusions among investigators. Efforts to develop quantitative biological metrics for evaluation of early life history studies should be continued [48-56]. It is evident that the use of quantitative measurements following procedures outlined in this paper led to statistically significant differences among treatments, whereas application of our best professional judgment assessment of semi-qualitative scoring procedures identified similar trends, but did not identify significant differences in any metric.
Acknowledgements
We thank Paul A Dinnel for providing oversight during initial phases of the laboratory study. We also thank Brian Hester, Tracy Schuh, Collin Ray, William Gardiner, and Mary Bacon for their expertise and dedication during the long days of the experiment. Support for this study was provided by Polaris Applied Sciences, Inc.
REFERENCES
- Douglas GS. Evaluation of environmental chemistry results associated with the NOAA report entitled “2008 Cosco Busan oil spill assessing toxic injury to Pacific herring embryos and larvae in San Francisco Bay”. New Fields Environmental Forensic Practice; Letter to Gary Mauseth, Polari Applied Sciences, Inc. 2009;124.
- NOAA/BML. The 2007 Cosco Busan oil spill: Assessing toxic injury to Pacific herring embryos and larvae in the San Francisco Estuary. Northwest Fisheries Science Center, National Marine Fisheries Service and NOAA. 2007.
- NOAA/BML. Standard operating procedures for the 2007 Cosco Busan oil spill: Assessing toxic injury to Pacific herring embryos and larvae in the San Francisco estuary. Draft report. North-west Fisheries Science Center, National Marine Fisheries Service, NOAA. 2007;43.
- Incardona JP, Carls MG, Day HL, et al. Cardiac arrhythmia is the primary response of embryonic Pacific herring (Clupea pallasi) exposed to crude oil during weathering. Environ Sci Technol. 2009;43:201-7.
- New Fields. Biological review of 2009 experimental data on Cosco Busan oil effects on herring, Part 1: Test and data validation. Report prepared for Polaris Applied Sciences, Inc. New Fields, Port Gamble WA, USA. 2009.
- New Fields. Biological review of 2009 experimental data on Cosco Busan oil effects on herring, Part 2: Evaluation of measured endpoints and relationship to potential causes. Report prepared for Polaris Applied Sciences, Inc. New Fields, Port Gamble WA, USA. 2009;78.
- New Fields. Laboratory demonstration of environmental factors and their effects on early stage development of Clupea pallasi. Report prepared for polaris applied sciences, Inc. New Fields, Port Gamble WA, USA. 2011;74.
- Wolf K. Experimental induction of blue sac disease. Trans Am Fish Soc. 1956;86:61-70.
- Marty G, Hose J, McGurk M, et al. Histopathology and cytogenetic evaluation of Pacific herring larvae exposed to petroleum hydrocarbons in the laboratory or in Prince William Sound, Alaska, after the Exxon Valdez oil spill. Can J Fish Aquat Sci. 1997;54:1846-57.
- Incardona JP, Carls MG, Day HL, et al. Cardiac arrhythmia is the primary response of embryonic Pacific herring (Clupea pallasi) exposed to crude oil during weathering. Environ Sci Technol. 2009;43:201-7.
- Hodson PV, Khan CW, Saravanabhavan G, et al. Alkyl PAH in crude oil cause chronic toxicity to early life stages of fish. Proceedings, 28th Arctic and Marine Oilspill Prog. Environ Sci Tech Div. 2007;10.
- Boudreau M, Sweezey MJ, Lee K, et al. Toxicity of orimulsion-400 to early life stages of Atlantic herring (Clupea harengus) and mummichog (Fundulus heteroclitus). Environ Toxicol Chem 2009;28:1206-17.
- Incardona JP, Vines CA. Cosco busan oil spill natural resource damage assessment: Data report of laboratory and field herring injury studies performed. 2008-9.
- Word JQ, Pinza MR, Word LS, et al. Biological review of 2009 experimental data on Cosco Busan oil effects on herring. Part 2: Evaluation of measured endpoints and relationship to potential causes. 2010.
- Alderdice DF, Velsen FPJ. Some effects of salinity and temperature on early development of Pacific herring (Clupea pallasi). J Fish Res Board Can. 1971;28:1545-62.
- Griffin FJ, Brenner MR, Brown HM, et al. Survival of Pacific herring larvae is a function of external salinity. Proceedings, Early Life History of Fishes in the San Francisco Estuary and Watershed, Santa Cruz, CA. 2004;37-46.
- Johnston IA, Vieira VLA, Temple GK. Functional consequences and population differences in the developmental plasticity of muscle to temperature in Atlantic herring Clupea harengus. Mar Ecol Prog Ser. 2001;213:285–300.
- Cherr GN, Pillai MC. Progress report: Environmental factors affecting reproduction and recruitment of Pacific herring in the San Francisco Estuary. Interagency Ecological Program for the Sacramento-San Joaquin Estuary. 1994.
- Dinnel PA, Hoover R, Lechuga L, et al. Development of larval Pacific herring, Clupea pallasi, bioassay protocols: refinement, validation, refinery effluent and cherry point ambient water testing during 2007. Final Report for Washington Department of Ecology by Shannon Point Marine Center, Western Washington University, Anacortes, WA. 2008;81.
- Hill J, Johnston IA. Photomicrographic atlas of Atlantic herring embryonic development. J Fish Biol. 1997;51:960-77.
- Dinnel PA, Middaugh DP, Schwarck NT, et al. Methods for conducting bioassays using embryos and larvae of Pacific herring, Clupea pallasi. Arch Environ Contam Toxicol. 2010.
- McIntosh S, King T, Wu D, et al. Toxicity of dispersed weathered crude oil to early life stages of Atlantic herring (Clupea harengus). Environ Toxicol Chem. 2010;29:1160-7.
- Carls MG, Rice JS, Hose JE. Sensitivity of fish embryos to weathered crude oil: Part I Low level exposure during incubation causes malformations, genetic damage and mortality in larval Pacific herring (Clupea pallasi). Environ Toxicol Chem. 1999;18:481-93.
- Pearson WH, Mokness E, Skalski JR. A field and laboratory assessment of oil spill effects on survival and reproduction of Pacific herring following the Exxon Valdez spill. American Society for Testing and Materials, Philadelphia (PA), USA. 1995.
- Dunnett CW. A multiple comparisons procedure for comparing several treatments with a control. J Am Stat Assoc. 1955;50:1096-121.
- SAS/STAT software. SAS and all other SAS Institute Inc. product or service names are registered trademarks or trademarks of SAS Institute Inc., Cary, NC, USA. 2008.
- Vines C, Robbins T, Griffin F, et al. The effects of diffusible creosote-derived compounds on development in Pacific herring (Clupea pallasi). Aquat Toxicol. 2000;51:225-39.
- Griffin FJ, Smith EH, Vines CA, et al. Impacts of suspended sediments on fertilization, embryonic development, and early larval life stages of the Pacific herring, Clupea pallasi. Biol Bull 2009;216:175-87.
- Linden O. Biological effects of oil on early development of the Baltic herring Clupea harengus membras. Mar Biol. 1978;45:273-83.
- Pearson WH, Woodruff DL, Kiesser SL, et al. Oil effects on spawning behavior and reproduction in Pacific herring (Clupea harengus pallasi). Final report to Environmental Affairs Department of the American Petroleum Institute, Washington DC, USA. 1985.
- Kocan RM, Hose JE, Brown ED, et al. Pacific herring (Clupea pallasi) embryo sensitivity to Prudhoe Bay petroleum hydrocarbons: Laboratory evaluation and in situ exposure at oiled and unoiled sites in Prince William Sound. Can J Fish Aquat Sci. 1996;53:2366-75.
- Heintz R, Short J, Rice S. Sensitivity of fish embryos to weathered crude oil: Part II. Increased mortality of pink salmon embryos incubating downstream from weathered Exxon Valdez crude oil. Environ Toxicol Chem. 1999;18:481-93.
- Pearson WH, Elston RA, Bienert RW, et al. Why did the Prince William Sound, Alaska, Pacific herring (Clupea pallasi) collapse in 1993 and 1994? Review of hypotheses. Can J Fish Aquat Sci. 1999;56:711-37.
- Carls MG, Marty D, Hose JE. Synthesis of the toxicological impacts of the Exxon Valdez oil spill on Pacific herring (Clupea pallasi) in Prince William Sound, Alaska, USA. Can J Fish Aquat Sci. 2002;59:153-72.
- Pearson WH, Deriso RB, Elston RA, et al. Hypothesis concerning the decline and poor recovery of Pacific herring in Prince William sound, Alaska. Rev Fish Biol Fish. 2011.
- Incardona JM, Collier T, Scholz N. Defects in cardiac function precedes morphological abnormalities in fish embryos exposed to polycyclic aromatic hydrocarbons. Toxicol Appl Pharm. 2004;196(2):191-205.
- Incardona JM, Carls M, Teraoka H, et al. Aryl hydrocarbon receptor-independent toxicity of weathered crude oil during fish development. Environ Health Perspect. 2005;113:1775-62.
- McDonald BG, Chapman PM. The need for adequate quality assurance/quality control measures for selenium larval deformity assessments: Implications for tissue residue guidelines. Integrated Environ Assess Manag. 2009;5:470-5.
- Strähle J, Jesuthasan S. Ultraviolet irradiation impairs epiboly in zebra fish embryos: Evidence for a microtubule-dependent mechanism of epiboly. Development. 1993;119:909-19.
- NOAA/BML. Standard operating procedures for the 2007 Cosco Busan oil spill: Assessing toxic injury to Pacific herring embryos and larvae in the San Francisco estuary. Draft report. Northwest Fisheries Science Center, National Marine Fisheries Service, NOAA. 2007;43.
- Berry JP, Gantar M, Gibbs PDL, et al. The zebra fish (Danio rerio) embryo as a model system for identification and characterization of developmental toxins from marine and freshwater microalgae. Comp Biochem Physiol Part C Toxicol Pharmacol 1956;145:61-72.
- Brinkman SF, Woodling JD, Vajda AM, et al. Chronic toxicity of ammonia to early life stage rainbow trout. Trans Am Fish Soc. 2009;138:433-40.
- Brinkworth LC, Hodson PV, Tabash S. CYP1A induction and blue sac disease in early developmental stages of rainbow trout (Oncorhynchus mykiss) exposed to retene. J Toxicol Environ Health. 2003;66:627-46.
- Adema-Hannes R, Shenker J. Acute lethal and teratogenic effects of tributyltin chloride and copper chloride on mahi mahi (Coryphaena hippurus) eggs and larvae. Environ Toxicol Chem. 2008;27:2131-5.
- Barron MG, Carls MG, Heintz R, et al. Evaluation of fish early life-stage toxicity models of chronic embryonic exposures to complex polycyclic aromatic hydrocarbons mixtures. Toxicol Sci 2004;78:60-7.
- Rosenthal H, Alderice DF. Sub lethal effects of environmental stressors, natural and pollution, on marine fish eggs and larvae. J Fish Res Bd Can. 1976;33:2047-65.
- Griffin FJ, Pillai MC, Vines CA, et al. Effects of salinity on sperm motility, fertilization, and development in the Pacific Herring, Clupea pallasi. Biol Bull. 1998;194:25-35.
- Pearson WH, Mokness E, Skalski JR. A field and laboratory assessment of oil spill effects on survival and reproduction of Pacific herring following the Exxon Valdez spill. American Society for Testing and Materials, Philadelphia (PA), USA. 1995.
- Pearson WH, Elston RA, Bienert RW, et al. Why did the Prince William Sound, Alaska, Pacific herring (Clupea pallasi) collapse in 1993 and 1994? Review of hypotheses. Can J Fish Aquat Sci. 1999;56:711-37.
- Pearson WH, Deriso RB, Elston RA, et al. Hypothesis concerning the decline and poor recovery of Pacific herring in Prince William Sound, Alaska. Rev Fish Biol Fish. 2011.
- SAS/STAT software. SAS and all other SAS Institute Inc. product or service names are registered trademarks or trademarks of SAS Institute Inc., Cary, NC, USA. 2008.
- Strähle J, Jesuthasan S. Ultraviolet irradiation impairs epiboly in zebra fish embryos: Evidence for a microtubule-dependent mechanism of epiboly. Development. 1993;119:909-19.
- US department of homeland security, US. Coast guard. Incident specific preparedness review (ISPR) M/V Cosco busan oil spill in San Francisco Bay. 2008.
- Vines C, Robbins T, Griffin F, et al. The effects of diffusible creosote-derived compounds on development in Pacific herring (Clupea pallasi). Aquat Toxicol. 2000;51:225-39.
- Wolf K. Experimental induction of blue sac disease. Trans Am Fish Soc. 1956;86:61-70.
- Word JQ, Pinza MR, Word LS, et al. Biological review of 2009 experimental data on Cosco Busan oil effects on herring. Part 2: Evaluation of measured endpoints and relationship to potential causes. 2010.