The peculiarities of Ag and Cu ions release from bactericidal textiles in water and their influence on spectral, electrical and biocide properties of textile
2 Institute of Urology of National Academy of Medical Science of Ukraine, Kyiv, Ukraine, Email: rudenko@gmail.com
3 Taras Shevchenko National University of Ukraine, Kyiv, Ukraine, Email: otananaiko@gmail.com
Received: 05-Jun-2018 Accepted Date: Jun 28, 2018; Published: 30-Jun-2018
Citation: Eremenko A, Petrik I, Rudenko A, et al. The peculiarities of Ag and Cu ions release from bactericidal textiles in water and their influence on spectral, electrical and biocide properties of textile. J Nanosci Nanomed. July-2018;2(1):20-24.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
In this paper, the dynamics of the release of silver and copper ions into water from bactericidal cotton fabrics (CF), modified by Ag NPs and bimetallic Ag/Cu particles (BMNPs) and physico-chemical properties of materials are studied. To determine the amount of released ions from the tissue surface modified by NPs, the samples of Ag-CF and Ag/Cu-CF were kept in water for 24 hours, after which the amount of ions desorbed from tissues was measured. These procedures were repeated every 24 hours for three days. The largest amount of ions (46%) was desorbed from the sample Ag-CF during first cycle, then 7, 7% after 2nd and 0, 7% after 3rd cycles. In case of Ag/Cu-CF, for 3 contact cycles of samples with an aqueous medium, 13%, 1% and 0.1% ions of silver in washout water respectively were obtained. Wherein 17% Cu2+ are released in first cycle, in the subsequent washout cycles, copper ions in water were not detected. The obtained results explain the high bactericidal activity of modified cotton textiles precisely in the first day of their use against a number of bacteria due to the active diffusion of both silver and copper ions that are formed on NPs’ surfaces when in contact with the humid environment. The textiles were investigated by UV-visible spectroscopy methods to determine the SPR position of NPs, the amount of metal on the fabric was determined by the flame atomic-absorption spectroscopy (FAAS) method, the correlation between the electrical conductivity of the tissues after each cycle of washing and the amount of NPs remaining after washing was investigated by measures of electrical resistance of the tissue. Comparison of biocide action of Ag NPs and BMNPs in the structure of tissues is studied. We study the effect of Ag-CF and Ag/Cu-CF on bacteria Escherichia coli, Klebsiella pneumoniae, Enterobacter aerogenes, Proteus mirabilis, Pseudomonas aeruginosa, and Staphylococcus aureus.
Introduction
Nanosized silver and copper particles (NPs) are known as effective antibacterial agents in relation to a wide range of pathogenic and opportunistic bacteria [1-5]. Nanosized bimetallic nanoparticles (BMNPs), which are formed by the combination of Ag and Cu NPs, in the colloidal solution, on the surface of silica, in polyelectrolyte films, as well as in tissues exhibit increased (as compared with their constituent) bactericidal activity [6,7].
There are numerous publications devoted to the introduction of silver NPs into fabrics: wool [8], cotton covered with hydrophobic polymer [9], viscose, polyamide [10]. The thermal introduction of Ag/Cu BMNPs into the fabric is convenient in terms of reducing the melting points of the components [11]. In this work it is shown that “a melting point depression in the range of 10-14 C was observed both for the one-pot synthesized Ag- Cu NPs and for a ‘‘Ag+ Cu mechanical mixture’’ of NPs, which are comparable to the corresponding results calculated by the CALPHAD method”.
Ag increases the oxidation activity of copper [12]. Due to substantial distinction of oxidizing potentials - 0.337 V for copper and 0.799 V for silver, copper facilitates the reduction of silver ions to NPs and conversely more reduction potential of silver ions will be reduced and copper will be oxidized [13]. Ag/Cu BMNPs have a higher bactericidal affectivity compared to their constituents [14,15]. The toxicity of the NPs impregnated with the tissue also reduces due to strong binding to the tissue structure compared with the use NPs in the solution. The depth of the penetration of the NPs in the skin is app. 30 angstrom [16].
Silver and its compounds are ionized in the presence of water and biological fluids. The silver ion easily interacts with proteins, amino acid residues, free anions, and receptors on membranes of living organisms. Toxics for bacteria are both nanoparticles and ions released from them [17-19]. As stated in the substantive review [20], there is a need to find the difference between the effects of the action of ions and Ag NPs in a microbial environment, taking into account that their location and mechanisms of action vary widely: the ion diffuses through biological barriers and achieves equilibrium concentration in most organs/cells, while the silver NPs are accumulated in specific cell/subcellular locations, after which, at their further dissolution, an extremely high local concentration of ions with a new effect is released [20]. Zhang et al. [21] emphasize the great difference in the quantitative estimation of ions localized on the surface of the textile and released in water, which can range from 1 to 45% by weight and depends on the washing cycles, or days of tissue healing in water. NPs and released ions of silver also generate reactive oxygen species (ROS) that kill bacteria [22-27]. The high affinity of ions for the biological receptors of microorganisms and their subsequent degradation causes the extremely high intensity of their fundamental and practical study.
Despite the large number of articles devoted to the study of the mechanisms of biocide action of silver and copper NPs in the tissues, the issues that are related to the dynamics of release of metal ions during the first hours and days of contact with the liquid medium-water and biological fluids, as well as the fixation of metals in the form of the NPs or their complete oxidation to the ions and the determination of the amount of metal on the surface of the tissues after the loss of a portion of the ions are not clear yet. In this paper, the dynamics of the release copper ions into water from bactericidal cotton fabrics (CF) modified by Ag NPs and of silver and BMNPs Ag/Cu is studied. Modified tissues were investigated by UV-visible spectroscopy methods to determine the state of the NPs by the shape and position of the SPR band, a flame atomic adsorption spectroscopy (FAAS) was used for quantitative evaluation of the metal on the fabric, and the electrical conductivity measurements to determine the effect of the amount of NPs remaining after washing, on the electrical resistance of the fabric. Comparison of biocide action of BMNPs depending on the anion of copper salt, namely nitrate or acetate, as a source of the corresponding NPs and their influence on bactericidal activity has been made.
Experiment
A simple and effective method for the production of cotton fabric modified with Ag NPs and BMNPs by means of a soft heat treatment without the use of chemical reducing agents and stabilizers, with an enhanced antibacterial effect compared with monoparticles Ag and Cu is presented in refs. [14,15,28]. The fabrics exhibit high bactericidal properties, but this method does not guarantee the particles formation with definite morphology and size; on the surface of the cotton, mainly 10-50 nm spherical particles, as well as silver and copper oxides, are formed.
1. Briefly, the technique for preparing bactericidal textile is as follows: Cotton fabric (CF)-madapolam, a density of 94 g/m2, AgNO3 and copper nitrate or acetate were used. 10 g of tissue was kept in 100 ml of salt solution with С=10-1M for 30 min. Ironing of the squeezed fabric at 200°C is accompanied by the appearance of yellow (in the case of Ag/CF) or yellow-brown (in the case of Ag/Cu/CF) color. Samples of tissue contained the ions of silver, copper and both ions Ag+/Cu2+ were prepared for comparison and dried during 24 h at room temperature on a paper substrate.
2. To determine the amount of released ions from the modified tissues, Ag/CF and BMNPs Ag/Cu/CF (0.12 g) were kept in 10 ml of water for 24 hours following which the amount of ions desorbed from tissues was analyzed. These procedures were repeated every 24 hours for three days. The concentration of copper ions has been determined by the absorption spectrum of complex [Cu (NH3)4]2+ at a wavelength of 638 nm. The amount of desorbed silver ions was determined by the appearance of SPR absorption band of the Ag NPs at 420 nm by adding NaBH4 to the ions solution. The absolute error of measurements of Ag+ and Cu2+ was 1.5 • 10-7 and 3.1 • 10-6 mol/g or 1.5 • 10-9 and 2.9 • 10-8 mol/cm2, respectively.
3. To measure the electrical resistance of the modified tissues, we used portable professional multifunction digital multimeter, HYELEC MS8229 (China). The electrode surface area was 1 mm2; the distance between electrodes during measurements was 1 cm; the surface area of the fabric was 4.0 cm2.
4. For measuring of metal content within tissue by the method of flaming atom-absorption spectroscopy (FAAS) the samples of fabric modified with metal NPs were treated in 10 ml of 1М HNO3 during twenty-four hours. The volume of the nitric acid was 25 ml. Content of metals in the obtained solution was measured by the flame atomic absorption spectrometry without additional sample pretreatment. A Carl Zeiss Jena AAS-N1 spectrometer equipped with hollow cathode lamps for copper and silver was used throughout this work. The equipment was operated at the standard conditions recommended by the manufacturer (wavelength: 324.7 nm (Cu); 328.1 nm (Ag); lamp current 6.0 mA; spectral slit width 0.2 nm). The acetylene flow rate and the burner height were adjusted in order to obtain the maximum absorbance signal. Nebulizer flow rate was 3.0 mL min-1. We have determined the amount of metal (Ag and Cu) on the surface of the fabrics (mkg\cm2) before and after their contact with water. The differences of these amounts can be used to calculate the amount of metal desorbed by water. On the results of the measuring the content of metals on the unit of the area of fabric has been calculated.
5. The diffusion-reflectance spectra (DRS) of the fabrics with NPs were registered by means of spectrophotometer Perkin Elmer Lambda Bio UVvisible with the integrating sphere of Labsphere RSA- PR- 20 in the range of waves 200-1000 nm.
6. The bactericidal properties of the samples were studied in the following way. In melted and cooled to 45°C meat-peptonic agar the suspension of test organisms containing 108 MK/ml was introduced and the mixture was poured into a petri dish. Test tissue samples with size 8 × 8 mm were putted on the surface of agar. Pure tissue that does not contain antimicrobial components was considered as control. Cultivations of bacteria were placed in thermostat at 37°C. The account of the results was performed after 24-48 hours for the largest area of growth inhibition (mm) microorganisms around the test sample. Experiments were repeated at least three times (subject to obtaining similar results). Antimicrobial activity against of pathogens namely Escherichia coli, Klebsiella pneumoniae, Enterobacter aerogenes, Proteus mirabilis, Pseudomonas aeruginosa and Staphylococcus aureus has been defined as inhibition zone in mm.
Results and Discussion
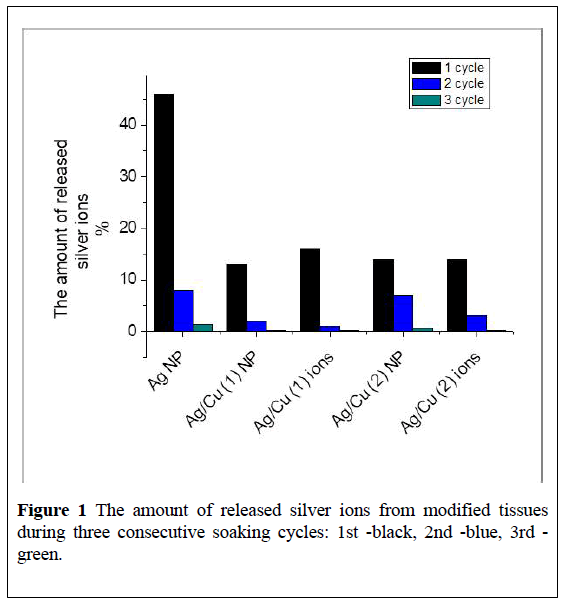
As noted above, the tissues passed three consecutive 24-hours soaking cycles in water, after each of that, the amount of released ions were determined. Specimens of tissues with metal NPs were designated as Ag (NP), Ag/Cu (1) (BMNPs obtained from silver and copper nitrates), Ag/Cu (2) (BMNPs derived from silver nitrate and copper acetate). Samples of tissues with ions of silver and copper are labeled Ag/Cu (1) "ions" and Ag/Cu (2) "ions". Figure 1 shows % of silver ions that are washed out from the corresponding tissue samples after 3 cycles of soaking.
It can be seen from the (Figure 1) that the largest amount of silver ions was washed out during the first day of soaking the tissue Ag (NPs) in water, after the second and third days of soaking, silver desorption decreases by almost 4, 5 times. Interestingly, the presence of copper in samples Ag/Cu (1) and Ag/Cu (2) very significantly contributes to the holding of silver within the fabric. Samples of tissues containing Ag+ and Cu2+ ions demonstrate a similar behavior (Figure 1).
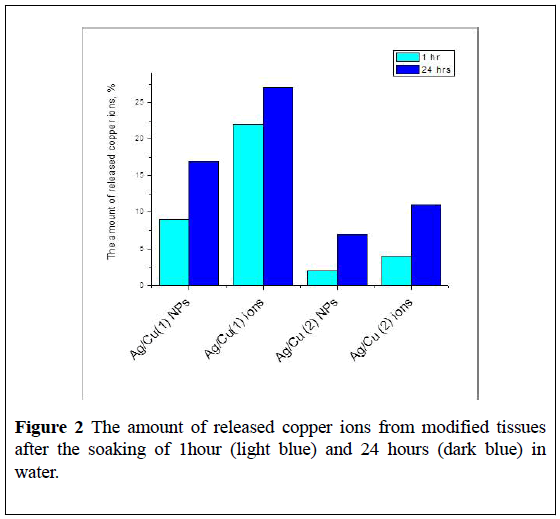
The amount of copper ions which were found in water after contact of tissues with Ag/Cu (1) and Ag/Cu (2) after 1 and 24 hours of soaking is much less than the number of desorbed silver ions (Figure 2). Copper on the surface of the tissue is mainly in the form of oxide and protoxide, from which the release of copper ions is not as active as in the case of Cu NPs. Possibly, Ag NPs firmly hold on the surface of copper oxides, with the formation of a structure similar to the alloy [14,15].
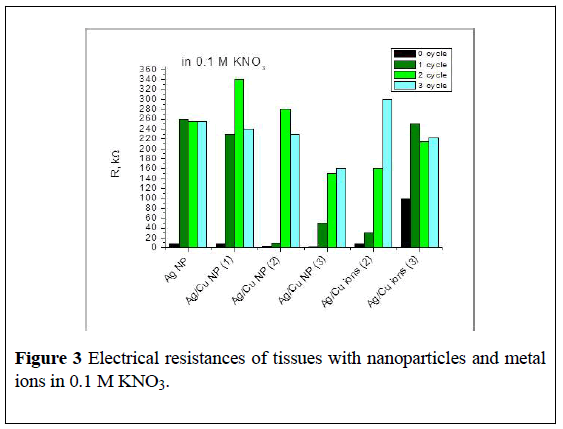
The second and third cycles of soaking (48 and 72 hours) do not lead to additional washing of copper ions from Ag/Cu (1) and Ag/Cu (2) fabrics. The same trend was observed for fabrics containing only ions of copper: about 30% of ions were washed out after 24 hours of contact with water. The concentration of metal particles in the tissue correlates with the electrical resistance (ER) of the samples.
Thus, the ER of BMNPs on dry fabric is 2700 kOhm, after 24 hours soaking in water and loss of metal ions it falls-120 kOhm, with an additional loss of metal ER increased: after 48 hours-520 kOhm, after 72 hours-1400 kOhm. After contact with the electrolyte, this line is respectively 50, 150 and 160 kOhm.
It should be noted that in some cases, in the presence of an electrolyte, BMNPS precipitated on the electrode surface.
Samples of tissues impregnated with silver and copper ions show the same tendency-a dry sample demonstrates the electrical resistance of 1000 kOhm, after 24, 48 and 72 hours of contact with water, it is 620, 1800 and 2000 kOhm respectively, after the measurements in the electrolyte, electrical resistance of Ag+/Cu2+ CF samples is approx. 30, 160 and 300 kOhm respectively (Figure 3).
Figure 3: Electrical resistances of tissues with nanoparticles and metal ions in 0.1 M KNO3.
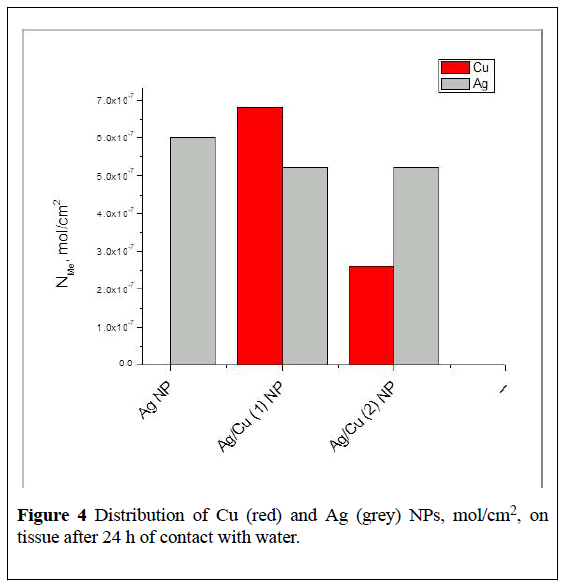
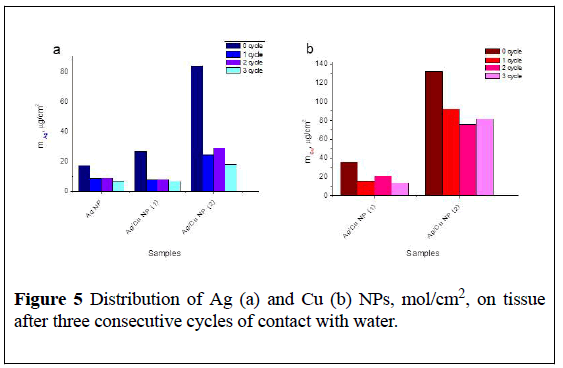
Figures 4 and 5 show data on the distribution of remaining copper and silver NPs per unit of tissue surface after release of corresponding ions during 1st cycle (24hrs) and 3cycles (24, 48 and 72hrs) of contact with water calculated according to FAAS measurements.
The amount of released ions depends on the metal composition introduces within tissue (Figure 5).
It is obvious that the strength of binding of NPs to the fabric and the composition of the particles formed during heat treatment depends on the initial copper salt-acetate or nitrate and its thermal stability during forming tissue’s bactericidal surface.
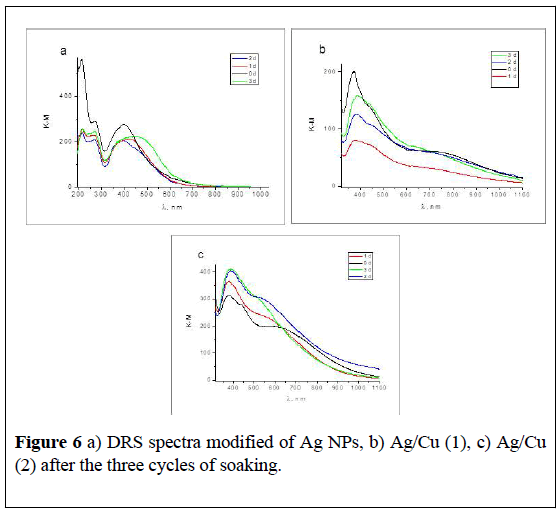
Decrease of the content of NPs on the surface of tissues after contact with water is clearly evident in the evolution of diffusion spectra (Figure 6).
The band of surface plasmon resonance of Ag NPs is observed for all samples. Maximum of SPR spectra are shifted after loss of the part of ions. The intensity of the SPR band of Ag NPs decreased, the shoulder in the range 400-600 nm could belong to the agglomerates of the NPs, or Ag2O (Figure 6a).
In Ag/Cu (1) (2) (Figure 6b and 6c), intensity the SPR of Ag decreased, after the second and third days of soaking it increases. We suppose that there is a recovery of silver and the formation of new Ag NPs from the silver ions with the participation of glucosidic tissue fragments.
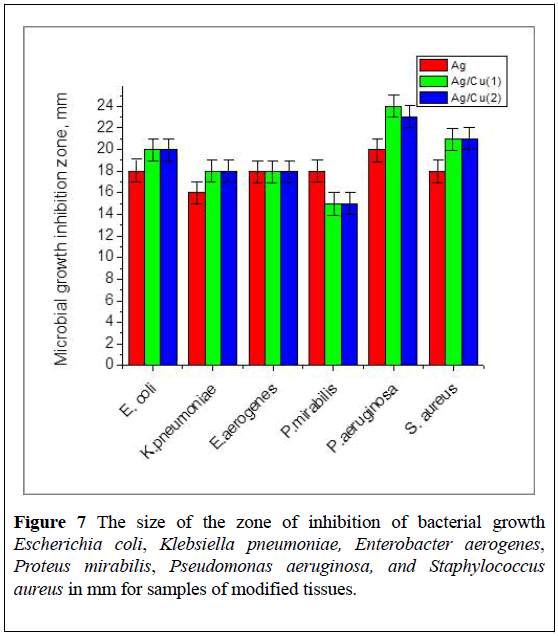
Bactericidal properties of modified tissue are shown in Figure 7.
The increased antimicrobial activity of BMNPs in tissue is a result of the released of bioactive binary ions Ag+/Cu2+ from the surface. The obtained results explain the high bactericidal activity of modified cotton textiles precisely in the first day of their use.
Conclusion
The dynamics of the release of silver and copper ions from bactericidal tissues, modified of Ag NPs and Ag/Cu BMNPs after 1, 2 and 3 days of soaking in water was studied. It was found that:
1) The largest number of Ag and Cu ions is released during the first 24 hours of contact of the tissue with the aqueous medium, after 48 hours of contact with water, the amount of released ions drops sharply, after 72 hours practically stopped.
2) Similar ion release behavior is shown by tissues modified with Ag+ and Ag+/Cu2+ ions, but their relative amount in the water is less than that of metallic bases.
3) It was found that in BMNPs the silver NPs are kept much stronger than within Ag/tissue and the concentration of Ag+ ions in water from such tissues are less. At the same time copper ions enter into water in a smaller quantity compared with silver ions and only in the first 24-hour cycle of soaking. The obtained results explain the high bactericidal activity of modified cotton textiles precisely in the first day of their use for disinfecting wound surfaces from a number of bacteria due to the active diffusion of silver and copper ions that are formed on their surfaces when in contact with the humid environment. The work is in the stage of further development.
REFERENCES
- Mner M, Sayar N, Yulug I, et al. Synthesis, characterization and antibacterial investigation of silver–copper nanoalloys. J. Mater. Chem. 2011;21:13150-5.
- Tan K, Kuan Y. Advances of Ag, Cu, and Ag-Cu alloy nanoparticles synthesized via chemical reduction route. J Nanopart Res. 2013;15:1537-29.
- Hemeg H. Nanomaterials for alternative antibacterial therapy. Int J Nanomedicine 2017;12:8211-14.
- Paszkiewicz M, Gołąbiewska A, Rajski L, et.al. Synthesis and characterization of monometallic (Ag, Cu) and bimetallic Ag-Cu particles for antibacterial and antifungal applications. J Nanomat. 2016;2016:2187940-11.
- Kim N, Shin K, Ijung I, et al. Ag-Cu bimetallic nanoparticles with enhanced resistance to oxidation: A combined experimental and theoretical study. J Phys Chem C 2014;118:26324-7.
- Taner-Camc M, Suzer S. Dynamic XPS measurements of ultrathin polyelectrolyt9e films containing antibacterial Ag-Cu nanoparticles. J Vac Sci Technol 2014;32:021510-4.
- Petrik I, Eremenko A, Smirnova N, et al. Synthesis and stabilization of Cu nanoparticles in aqueous solutions and their bactericidal activity. Chem Phys and Tech of Surf. 2014;5:74-8.
- Mura S, Greppi G, Malfatti L, et al. Multifunctionalization of wool fabrics through nanoparticles: A chemical route towards smart textiles. J Colloid Interface Sci. 2015;456:85-7.
- Jasiorski M, Leszkiewicz A, Brzezinski S, et al. Textile with silver silica spheres: its antimicrobial activity against Escherichia coli and Staphylococcus aureus. J Sol-Gel Sci Technol. 2009;51:330-4.
- Bianco C, Kezic S, Svetlicic MCV, et al. In-vitro percutaneous penetration and characterization of silver from silver-containing textiles. Int J of Nanomedicine. 2015;10:1899-9.
- Delsante S, Borzone G, Novakovic R, et al. Synthesis and thermodynamics of Ag-Cu nanoparticles. Phys. Chem. Chem. Phys. 2015;17:28387-25.
- Hai HT, Takamura H, Koike J. Oxidation behavior of Cu-Ag core-shell particles for solar cell applications. J. Alloy Compd. 2013;564:71-7.
- Skoog DA, West DM, Holler FJ, et al. Fundamentals of analytical chemistry 9th edn. Brooks/Cole 2014:1090.
- Petrik I, Eremenko A, Rudenko A. Enhanced bactericidal activity of cotton fabrics modified by binary Ag/Cu nanoparticles. World J of Biochem Mol Biol. 2018;3:1-5.
- Eremenko A, Petrik I, Smirnova N, et al. Antibacterial and antimycotic activity of cotton fabrics, impregnated with silver and binary silver/copper nanoparticles. Nanoscale Res Lett. 2016;11:28-9.
- Yuranova T, Rincon A, Pulgarin C, et al. Performance and characterization of Ag-cotton and Ag/TiO2 loaded textiles during the abatement of E. coli. J Photochem Photobiol. 2006;181:363-6.
- Levard C, Mitra, S, Yang T, et al. Effect of chloride on the dissolution rate of silver nanoparticles and toxicity to E. coli. Environ Sci Technol. 2013;47:5738-7.
- González AG, Mombo S, Leflaive J, et al. Silver nanoparticles impact phototrophic biofilm communities to a considerably higher degree anionic silver. Environ Sci Pollut Res Int. 2015;22:8412-24.
- Tlili A, Cornut J, Behra R, et al. Harmful effects of silver nanoparticles on a complex detrital model system. Nanotoxicology 2016;10:728-7.
- Ziye Xiong. Ag-Cu bimetallic nanoparticle synthesis and properties by university of pittsburgh 2017.
- Zhang W, Yao Y, Sullivan N, Chen Y. Modeling the primary size effects of citrate-coated silver nanoparticles on their ion release kinetics. Environ. Sci. Technol. 2011;45:4422-6.
- Kittler S, Greulich C, Diendorf J, et al. Toxicity of silver nanoparticles increases during storage because of slow dissolution under release of silver ions. Chem Mater. 2010;22:4548-6.
- Glover R, Miller J, Hutchison J, et al. Generation of metal nanoparticles from silver and copper objects: Nanoparticle dynamics on surfaces and potential sources of nanoparticles in the environment. ACS Nano. 2011;5:8950-7.
- Zhai Y, Hunting E, Wouters M, et al. Silver nanoparticles ions and shape governing soil microbial functional diversity: Nano shapes micro, Front Microbiol. 2016;7:1123-9.
- Reidy B, Haase A, Luch A, et al. Mechanisms of silver nanoparticle release, transformation and toxicity: A critical review of current knowledge and recommendations for future studies and applications. Materials. 2013;6:2295-5.
- Liu J, Sonshine D, Shervani S, et al. Controlled release of biologically active silver from nanosilver surfaces. ACS Nano. 2010;4:6903-10.
- Suresh A, Pelletier D, Wang W, et al. Cytotoxicity induced by engineered silver nanocrystallites is dependent on surface coatings and cell types. Langmuir. 2012;28:2727-8.
- Eremenko А, Petrik I, Smirnova N, et.al. Bactericidal cotton fabrics modified by silver and copper nanoparticles: optical spectra, structures, electrical resistance. J Anal, Bioanal Sep Tech. 2016;1:1-5.
Keywords
Nanoparticles; bactericidal fabrics; ion release; silver; copper; bacteria