The relationship between the prolapse stage and pelvic diameters for women with urinary incontinence
2 Afyon Kocatepe University, Afyon, Turkey
3 Department of First and Emergency, Nigde Zubeyde Hanım Vocational School, Omer Halisdemir University, Nigde, Turkey
4 Department of Therapy and Rehabilitation, Kozaklı Vocational School, Nevşehir Hacı Bektası Veli University, Nevsehir, Turkey
5 Bozok University School of Medicine, Yozgat, Turkey
Received: 19-Jan-2018 Accepted Date: Jan 28, 2018; Published: 14-Feb-2018, DOI: 10.37532/1308-4038.18.11.20
Citation: Susar H, Aycan K, Ertekin T, et al. The relationship between the prolapse stage and pelvic diameters for women with urinary incontinence. Int J Anat Var. 2018;11(1):020-023.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
The Urinary incontinence is the involuntary urine loss causing a social and hygienic problem. The problem of urinary incontinence is often associated with pelvic prolapse. In our study we aimed to reveal the anatomical features of organs or formations in women who have pelvic prolapse together with urinary incontinence, and to evaluate the relationship between illness and age. This study was carried out on female patients who complained of urinary incontinence due to enforcement of the Urogynechology Policlinic of Erciyes University Faculty of Medicine between June 2006 and September 2007. Diameters of the aperture pelvis inferior (diameter sagittalis, diameter transversa) and aperture pelvis superior (diameter anatomica, diameter diagonalis) were measured from MRI scans of 46 cases of women who had urinary incontinence and prolapse, and whose prolapse were staged according to POPQ (Pelvic Organ Prolapse Quantification) system. The lengths of the pelvic diameters of the cases were on average 10.93 ± 0.96 cm for diameter sagittalis, 10.03 ± 0.86 cm for aperture pelvis inferior- diameter transversa, 12.00 ± 0.88 cm for diameter anatomica length and 12.89 ± 0.96 cm for diameter diagonalis. There was a statistically significant relationship between diameter sagittal lengths and prolapse stages in our study (p<0.05). The conclusion is that this increase in the sagittal diameter can weaken the pelvic floor and pave the way for pelvic diseases.
Keywords
Urinary incontinence; Prolapse; Pelvic diameter; Bladder
Introduction
Urinary incontinence is the involuntary urine loss causing a social and hygienic problem [1,2]. The prevalence in various studies ranges from 2.9% to 50% [1,3]. The problem of urinary incontinence is often associated with pelvic prolapse [4,5]. The urinary system consists of kidneys that filter urine from the blood, ureters that transmit urine from the kidneys to the bladder, the bladder that collects this urine, and urethra that ejaculates the urine accumulating in the bladder out of the body [6]. At rest, the pressure in the urethra is higher than the pressure in the bladder. On the other hand, this situation is reversed in micturition. Intravesical and intraurethral pressure relations at rest should not change even if intraabdominal pressure increases. If this pressure is not in the desired direction, urinary incontinence (UI) will occur. The emergence of this pattern can be caused by anatomical and functional disorders in the muscular and nervous system [7,8]. UI is a common disease affecting the quality of life of millions of people around the world in a bad way [9]. Situations such as pelvic stenosis during birth, forceps births or many normal births may cause severe damage to the urogenital organs and weakness of the muscle and connective tissues in the bladder. Because of such reasons, the sphincter mechanism of the bladder becomes insufficient and UI may develop. With the UI, downward sagging of the anterior wall of the vagina or the sagging of the bladder into the vagina (cystocele) may also be seen [10]. The pelvic diameter in women with incontinence may change with age and the sacrum rotates forward to make its mass more horizontal. This rotational movement of sacrum increases the distance between os coccyx and sympyhsis pubis, and the pelvic depth. Increased pelvic diameters due to congenital or biomechanical reasons cause enlargement of the pelvic floor. The enlargement in the pelvic floor increases prolapse susceptibility by reducing the support of pelvis [11]. Pelvic organ prolapse was detected in 50% of women with UI complaints [12]. Pelvic prolapse is defined according to the organ affected or displaced as a primer in diseases where the pelvic support is impaired. Pelvic Organ Prolapse Quantification (POPQ): After the entire prolapsing structures in the POPQ method were evaluated, staging was performed according to the portion showing the most prolapse. Criteria used in staging are as follows: Stage 0: No prolapse. Stage 1: Distal of prolapsing structures showing prolapse is 1 cm above the hymen level. Stage 2: Distal of prolapsing structures showing prolapse is at 1 cm proximal or distal of the hymen level. Stage 3: The most distal of the prolapsing structures is more than 1 cm below the hymen level, but there is no eversion in the vagina. In Stage 4, there is eversion in the vagina. The distal of the prolapsing structures sagged more than a distance corresponding to about 2 cm inward from the total vaginal length [13]. Coronal and sagittal MRI scans were used to evaluate the pelvic floor anatomy of the patients who underwent pelvic prolapse staging. On MRI scans, measurements of diameter anatomica, diameter diagonalis and aperture pelvis inferior belonging to the aperture pelvis superior, and diameter transversa and diameter sagittalis belonging to the aperture pelvis inferior were performed. The weakness of m. levator ani weakens the pelvic diaphragm and prepares the ground for pelvic prolapse.
In our study we aimed to reveal the anatomical features of organs or formations in women who have pelvic prolapse together with urinary incontinence, and to evaluate the relationship between illness and age.
Methods
Study groups
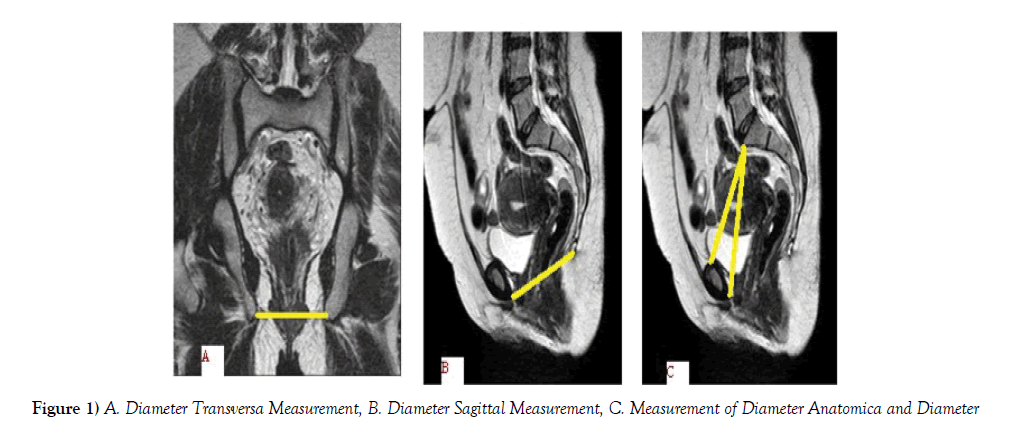
This study was carried out with the approval of Erciyes University Medical Faculty Ethics Committee (09/38). Our study was conducted on 46 women cases who applied to Erciyes University Faculty of Medicine Urogynecology Polyclinic. Cases were not studied that previous surgical for pelvic organs. There were complaints of urinary incontinence with pelvic prolapse in the cases taken to the study. The relationship between POPQ stages and diameters was evaluated. Diameters of the aperture pelvis inferior (diameter sagittalis, diameter transversa) and aperture pelvis superior (diameter anatomica, diameter diagonalis) were measured from MRI scans of 46 cases of women (Figure 1A-C).
The mean age of the cases participating in our study was 49.58 ± 10.58 (It was between 31 and 80).
Statistical analysis
Measurements were evaluated using ‘SPSS for Windows 15.0’ statistical program. The Shapiro-Wilk test was used to look for normal distribution. In comparison of groups; the in normal distributions variance analysis and in non-normal distributions Kruskal-Wallis test was used (p<0.05 was considered significant).
Results
The minimum age was 31 years and the maximum age was 80 years in the 46 cases accepted to the study; and the mean age was calculated to be 49.58 ± 10.58 (Table 1).
| Number | Minimum | Maximum | Mean | Standard Deviation | |
|---|---|---|---|---|---|
| Age | 46 | 31 | 80 | 49.58 | ± 10.58 |
| Diamater Sagittalis (cm) | 46 | 9 | 13 | 10.93 | ± 0.96 |
| Diamater Transversa (cm) | 46 | 8.5 | 12.2 | 10.03 | ± 0.86 |
| Diamater Anatomica (cm) | 46 | 9 | 13.5 | 12 | ± 0.88 |
| Diamater Diagonalis (cm) | 46 | 9.9 | 15.2 | 12.89 | ± 0.96 |
Table 1: Age and pelvic diameter measurements of patients
As a result of the measurements, there was no statistically significant relationship between the prolapse stage and age (p=0.157). No patients were found to be in the 4th stage of prolapse (Table 2).
| Prolapse stage | Number | Mean Age | Standartd Deviation |
|---|---|---|---|
| 0 | 13 | 50.92 | ± 10.51 |
| 1 | 13 | 44.92 | ± 9.81 |
| 2 | 13 | 49.53 | ± 8.19 |
| 3 | 7 | 55.85 | ± 13.96 |
Table 2: Relationship between age and the prolapse stages
There was no statistically significant relationship between the prolapse stage and the lengths of aperture pelvis inferior and diameter transversa (p=0.489) (Table 3).
| Prolapse stage | Number | Mean diameter length (cm) | Standard Deviation |
|---|---|---|---|
| 0 | 13 | 9.6 | ± 9.17 |
| 1 | 13 | 10 | ± 9.35 |
| 2 | 13 | 10 | ± 9.37 |
| 3 | 7 | 10.3 | ± 9.92 |
Table 3: Relationship between diameter transversa and prolapse stages
In 7 cases with prolapse stage 3, the mean length of the aperture pelvis inferior and diameter sagittalis was 10.98 cm, and there was a statistically significant relationship between prolapse stage and sagittal diameter lengths (p=0.007). A statistically significant relationship was found between the cases with stage 0, who do not have prolapse, and prolapse stage 1 and 2 (p=0.007). Sagittal diameter increased as the prolapse stage increased. There was no statistically significant relationship between prolapse stage 1 and 2, prolapse stage 2 and 3, prolapse stage 1 and 3, and prolapse stage 0 and 3 (Table 4).
| Prolapse stage | Number | Mean diameter length (cm) | Standard Deviation |
|---|---|---|---|
| 0 | 13 | 10.23 | ± 0.79 |
| 1 | 13 | 11.1 | ± 0.67 |
| 2 | 13 | 11.45 | ± 1.04 |
| 3 | 7 | 10.98 | ± 0.96 |
Table 4: Relationship between diameter sagittal and prolapse stages
When we compared the patients’ prolapse stages with the diameter diagonalis lengths:
No statistically significant relationship between diameter diagonalis and prolapse stage was found (p=0.315) (Table 5).
| Prolapse stage | Number | Mean diameter length (cm) | Standard Deviation |
|---|---|---|---|
| 0 | 13 | 12.2 | ± 1.04 |
| 1 | 13 | 12.6 | ± 1.17 |
| 2 | 13 | 11.9 | ± 1.07 |
| 3 | 7 | 12 | ± 0.03 |
Table 5: Relationship between diameter diagonalis and prolapse stages
There was not a statistically significant relationship between prolapse stage and diameter anatomica (p=0.168) (Table 6).
| Prolapse stage | Number | Mean diameter length (cm) | Standard Deviation |
|---|---|---|---|
| 0 | 13 | 12.76 | ± 1.03 |
| 1 | 13 | 13.39 | ± 1.04 |
| 2 | 13 | 12.73 | ± 0.84 |
| 3 | 7 | 12.52 | ± 0.66 |
Table 6: Relationship between diameter anatomica and prolapse stages
Discussion
The age range of 46 cases was found to be in a wide range between 31 and 80. Urinary incontinence and pelvic prolapse, which are the clinical reflection of pelvic floor dysfunction, is a clinical picture commonly encountered among women. They have become an epidemic disease that causes thousands of women worldwide to suffer from social, physical and psychological wellbeing. Recent studies have shown that the UI influences about two hundred million people around the world. Pelvic organ prolapse is seen in 38% of women with UI complaints [14]. Pelvic organ prolapse affects more than half of women over the age of fifty [15]. In 2001, Britain alone spent $ 1.012 million for pelvic organ prolapse surgery. Studies have shown that both illnesses occur and can be prevented at later ages [16].
Tosunoglu et al. investigated 400 cases of Turkish women in their study titled ‘Pelvic organ prolapse frequency in women over 40 years old’. Pelvic organ prolapse was detected in 65 of the cases studied. While the mean age of the women with prolapse was 57 ± 12.3, the mean age of the cases in our study was 49.58 ± 10.58 [17].
Aytan et al. examined 1354 Turkish women cases in their study titled ‘Prevalence of pelvic organ prolapse and related factors in a general population of women’. Of these, 358 were diagnosed with pelvic prolapse and the mean age was 42.89 ± .4. The average age revealed by this study, which was again conducted among Turkish women, was lower than the average age of women with prolapse in our study [18].
In their study, entitled ‘Self-cut polypropylene mesh use in the treatment of urinary incontinence and pelvic organ prolapse’ Onol et al. found that the mean age of 89 patients with incontinence and pelvic organ prolapse was 51.9 years. The mean age of the cases in this study and the age of the cases in our study are similar [19].
Stav et al. found an average age of 55.5 years (age range 19-90) when they studied the anatomical structures of pelvic bones and muscles in Israeli women and their relationships with UI. The average age of women who experienced problems about UI was 60.97 years, while the average age of the control group was 50.77 (p<0.0001) [10]. The average age of 46 cases in our study is 49.58 (31-80). According to our findings, it was understood that Turkish women had lower age of incidence of incontinence than Israeli women. It is therefore possible to arrive at the opinion that the age of incidence of illness can vary according to race.
Kohli N et al. evaluated computerized tomography results in order to compare the bone pelvis sizes between women with and without prolapse. 34 women with vaginal prolapse and 34 women without vaginal prolapse were included in the study. In the study, pelvis entry, anterior-posterior diameter and transversa diameter, pelvic exit-transversa diameter and anteriorposterior diameter were measured. In this study, the mean age of women was 63.4 ± 8.3. In the control group cases, the mean anterior-posterior diameter of pelvic exit was reported to be 12.5 cm and the mean interspinous diameter was reported to be 11.5 cm. They also found that the same diameter measurements were similar in women with prolapse. In addition, they showed that the diameter transversa, which is the inner diameter of pelvis, was significantly larger in the prolapse group than the control group (p=0.006). In our study, the sagittal diameter of women without prolapse was 10.23 cm; and we measured the sagittal diameter of women with prolapse in the third stage as 11.45 cm. We found that the sagittal diameter increased as the prolapse stage increased [20].
Tamara et al. found that pelvic diameters were close to each other in female cases with pelvic organ prolapse (42 cases) and without (42 cases) [21].
In the study where they evaluated the pelvic diameter measurements of women with m. levator ani defect, Burger et al. made pelvic diameter measurements using MRI scans of women without the m. levator ani defect in 99 cases of women (control group) and with bilateral severe m. levator ani defect in 50 cases. Diameter interspinosus and diameter transversa in the control group were 10.7 ± 0.8 cm, and 11.2 ± 1.0 cm, respectively. The same measurements were 10.6 ± 0.7 cm and 11.1 ± 0.8 cm in the cases with muscle defect. They did not find any statistically significant difference between measurements. The measurement results show similarity to the pelvic diameters of our study [22].
In the study where Handa et al. in 2013 measured the pelvic diameters of 35 cases using three-dimensional imaging in women with and without pelvic floor disease, the mean age of the cases with pelvic floor disease is 49.0 and is similar to the result in our study. Diameter interspinosus, diameter conjugate and diameter transversa of cases with pelvic floor disease were calculated to be 10.7 cm, 11.7 cm and 13.2 cm, respectively. They also reported in study that the diamater transversa is wider than normal dimensions. In our study, the diameter transversa was close to normal values [23].
As a result of the literature study, a study was encountered, of which prolapse was staged using the POPQ method as well as the relationship between pelvic diameters and the stages was evaluated. In this study, Ran Li et al. evaluated the relationship between the clinical levels of prolapse and pelvic diameter-m. levator ani. In the study, they reported that there was no statistically significant difference between the prolapse stages and pelvic measurements as well as between pelvic measurements of the control group and prolapse groups [24]. In our study, there was a statistically significant relationship between the cases with prolapse stage 3 and diameter sagittalis, and as the prolapse stage increased, the sagittal diameter has also increased.
As a result, the increase in the sagittal diameter can lead to increase in the susceptibility to pelvic floor diseases by weakening the pelvic floor.
REFERENCES
- Aksoy H. Transobturator Tape Application and Short Term Results in Stress Incontinence Therapy. Specialty Thesis, Institute of Health Sciences, Taksim Education and Research Hospital Women’s Disease and Birth Clinic, Istanbul. 2006; pp: 31.
- Zizzi PT, Trevisan KF, Leister N, et al. Women’s pelvic flor muscle strength and urinary and anal incontinence after childbirth: a cross-sectional study. Rev Esc Enferm. 2017;51:1-8.
- Oversand S, Staff A, Sandvick L, et al. Levator ani defects and the severity of symptoms in women with anterior compartment pelvic organ prolapse. Int Urogynecol J. 2018;29:63-9.
- Bureau M, Carlson KV. Pelvic organ prolapse: a primer for urologists. Can Urol Assoc J. 2017;11:125-8.
- Yalcın O, Delier H. Urinary incontinence and pelvic organ prolapse: diagnosis and treatment choice. Turkish Society of Gynecology and Obstetrics. 2004;8:198-200.
- Elhan A, Arıncı K. Anatomi I, Guneş Medicine Bookstores. 2006;317-22.
- Atasu T, Saymay S. Jinekoloji Kadın Hastalıkları. Nobel Medicine Bookstores. 2001; pp: 595.
- Ciftci O, Gunay O. The Factors Affecting Urinary Incontinence Psychosis in Women Who Attended the Gynecology Clinic of Kayseri Education and Research Hospital. Erciyes Med J. 2011;33:301-8.
- Patrick C. Walsh. Campbell Uroloji II. Gunes Medicine Bookstores. 2005;1027-30.
- Stav K, Alcalay M, Peleg S, et al. Pelvis architecture and urinary incontinence in women. Eur Urol. 2007;52:239-44.
- Sakala E. Obstetrics and Gynecology. 1999; pp: 224.
- Khullar V, Anding R, Robinson D, et al. Under what circumstances should stress incontinence surgery be performed at the same time as prolapse surgery?. Neurourol Urodyn. 2017,36:909-14.
- Bulut Comu N. Correlation of magnetic resonance findings on the pelvic floor with prolapsus phase urinary incontinence. 2008;1-18.
- Tanrıverdi HA, Hakan S. Epidemiology, etiology and risk factors of urinary incontinence and pelvic prolapse. Turkey Clin J Gynecol Obstetrics. 2004;14:231-8.
- Mendes A, Hoqa L, Gonçalves B, et al. Adult women’s experiences of urinary incontinence: a systematic review of qualitative evidence. JBI Database system Rev Implement Rep. 2017;15:1350-1408.
- Seven M, Akyuz A, Acıkel C. Validity and reliability study of urogenital prolapse quality of life scale. Preventive Medicine Bulletin, 2008;38:595-608.
- Canan S, Mustafa T, Teksin C. Examination of estrogen receptor and collagen type IV expression in vaginal and sacrouterine ligaments in cases with pelvic organ prolapse and urinary incontinence. J Turkish Obst Gynecol Soc. 2008;5:123- 9.
- Tosunoglu D. Examination of pelvic organ prolapse frequency and its effects on life in women over 40 years of age. Graduate Thesis. 2010;35-7.
- Onal F, Avcı E, Ergonenc T. Use of cut polypropylene mesh in the treatment of stress urinary incontinence and pelvic organ prolapse. Turkish Urol Ass. 2009;2:117-23.
- Sze EH, Kohlı N, Miklos JR, et al. Computed tomography comporison of bony pelvis dimensions between women with and without genital prolapse obstet gynecol. Obstetrics and gynecology. 1999;93:229-32.
- Stein TA, Kaur G, Summers A, et al. Comparison of bony dimensions at the level of the pelvic flor in women with and without pelvic organ prolapse. Am J Obstet Gynecol. 2009;200:241.
- Berger MB, Doumouchtsis SK, Delancey JO. Are bony pelvis dimensions associated with levator ani defects? A case- control study. Int Urogynecol J. 2013;24:1337-83.
- Brown KM, Handa VL, Macura KJ, et al. Three- dimensional shape differences in the bony pelvis of women with pelvic flor disorder. Int Urogynecol J. 2013;24:431-9.
- Li R, Song Y, Ma M. Relationship between levator ani and bony pelvis morphology and clinical grade of prolapse in women. Clinic Anatomy. 2015;28:813-9.