The role of endoscopic decompression of the optic chiasm and the optic nerve for skull base meningioma: A short review of practice
2 Department of Radiology, Aberdeen Royal Infirmary, Aberdeen, Scotland
Received: 22-Aug-2018 Accepted Date: Oct 06, 2018; Published: 10-Oct-2018
Citation: Singh K, Kanodia AK, Kamel MH, et al. The role of endoscopic decompression of the optic chiasm and the optic nerve for skull base meningioma: A short review of practice. Neurosurg J. 2018;1(1): 17-20.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Skull base meningiomas are typically slow growing and often present late only once they begin to compress nearby neurovascular structures. While craniotomy has been the mainstay of treatment in the past, endoscopic endonasal trans-sphenoidal (EET) techniques are increasingly favoured due to being less invasive with fewer complications. This short review analyses the experience of two neurosurgical centres using an endoscopic approach to solely decompress the optic apparatus where complete meningioma resection was considered too great a risk. Cases were retrospectively analysed from electronic medical and operative records from June 2008 to December 2017. Patients were selected if they had undergone EET surgery to debulk a meningioma causing deterioration of their vision but were deemed unsuitable for total resection due to tumour size or the relationship with surrounding neurovascular structures. 196 patients underwent endoscopic transsphenoidal surgery primarily for pituitary tumours in this 114-month period. Of these cases, 4 patients underwent EET surgery solely to debulk a meningioma compressing their optic apparatus (M=3, F=1) with a median age of 56.5 years (range 53-69 years). Half reported some improvement in their visual field perception, while one case reported a benefit in both visual fields and acuity. None of the cases described any post-operative deterioration in their sight. This short review of cases demonstrates our experience of the safety and efficacy of an EET approach for decompression of the optic nerve and chiasm. This supports its ongoing use in the protection of key neurovascular structures at risk of compromise by an expanding skull base meningioma.
Keywords
Endoscopic; Transsphenoidal; Meningioma; Optic chiasm; Optic nerve
Meningiomas make up nearly a quarter of all tumours located intracranially [1]. Nearly half arise from the base of the anterior, middle or posterior skull fossae, with the anterior fossa being the most common site within the skull base [2]. Growth rates are slow in the majority of cases, with the exception of rare highly invasive forms, but over time these tumours can develop into extremely large lesions [3]. Those arising from the anterior fossa can develop and extend into the orbit, paranasal sinuses or infratemporal fossa and their slowly progressive nature means that symptoms present when the tumour has grown to a sufficient size to cause mass displacement or disruption of intracranial neurovascular structures. These tumours have low recurrence rates if complete resection is achieved, although their indolent nature often results in a delayed diagnosis [4-7]. When discovered, these lesions can be large in size and spread out, making surgical resection challenging – especially when near or enveloping the cranial nerves located in the skull base [8].
While anterior cranial base meningiomas have traditionally been managed via craniotomy using several different approaches, the endoscopic endonasal trans-sphenoidal (EET) approach has become increasingly adopted owing to its less invasive nature and reduced postoperative morbidity [9-11]. This approach allows surgeons to achieve direct access to the tumour while avoiding manipulation of key regional neurovascular structures such as the optic nerve. Unlike a craniotomy, endoscopic decompression of the optic nerve allows early visualisation of the optic nerve as it exits the orbit and at the optic chiasm, thus reducing the risk of inadvertent damage. In this short report, we describe the use and outcomes of EET in decompressing the optic nerve and optic chiasm specifically for those patients whose skull base meningiomas are unsuitable for total resection. This may be due to their size or their relationship with nearby critical structures such as the cavernous sinus and the neurovascular structures contained within (i.e. the internal carotid artery and cranial nerves III, IV, V1, V2 and VI), besides optic nerves, chiasm, optic tracts and anterior cerebral arteries.
Methods
Patient cohort
We reviewed the records of all patients who underwent an endoscopic endonasal resection of a brain tumour from June 2008 to December 2017. Records were analysed from two neurosurgical centres in the North east of Scotland (Ninewells Hospital in Dundee and Aberdeen Royal Infirmary) responsible for providing services to a combined population of nearly 1 million patients [12,13]. Cases were selected for the purposes of this report if they were determined to have had a basal meningioma that threatened their vision but due to its size or the tumour engulfing nearby neurovascular structures (e.g. the internal carotid artery etc.), was deemed unsuitable for complete resection. Thus, we are identifying the outcomes of patients who were undergoing endoscopic surgery with the sole purpose of optic chiasm/canal decompression to either stabilise or improve their sight whose tumours were deemed too complex for complete resection. By focussing on this subset, an assessment of the safety and efficacy of a ‘vision preserving’ approach only in these patients with surgically complex meningiomas could be performed. Surgery was performed via a transsphenoidal approach with the modified (“rescue”) nasoseptal flap pedical transposition technique using the Karl-Storz Spies ClaraTM and Chroma® Visualization Enhancement Tools system [14].
Data collection
For the identified cases, pre-operative and post-operative ophthalmological examinations, operative reports and electronic hospital records were also analysed and documented. Visual acuity was tested via simple Snellen charts while formal visual fields testing was performed using an automated visual field analyser. Radiological imaging was reviewed to determine the extent of optic nerve decompression, as well as the post-operative anatomy of the spheno-ethmoidal region. Data were also collected relating to the underlying pathology as well as basic demographic data. Finally, the patients’ immediate and delayed post-procedure course was also reviewed, analysing the follow-up duration as well as pituitary endocrine function post-operatively. Ethical approval was sought through the Caldicott Guardian to access patient records.
Results
Patient characteristics
Over the 114-month period, 196 patients underwent endoscopic transsphenoidal procedures, primarily for pituitary adenomas. Within this cohort, 4 patients (M=3, F=1) underwent EET debulking of a meningioma threatening their optic chiasm and/or optic nerve, where total resection had been ruled out. Median age for these patients was 56.5 years old (range 53-69 years) and the pre-operative co-morbidity burden was similar across all 4 cases with a median Charlson Co-Morbidity Index of 4 (range 3-5) cases summarised in Table 1. All had reported worsening vision due to the compression of the optic neural structures and verified by visual acuity and visual field assessment by ophthalmology.
| Case | Age (yrs) | Gender | Follow-up (Months) | Co-morbidities | Pre-operative vision | Post-operative vision | Pituitary function | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Visual acuity | Visual fields | Visual acuity | Visual fields | Pre-op | Post-op | |||||||||
| Right | Left | Right | Left | Right | Left | Right | Left | |||||||
| 1 | 53 | F | 51 | Transient hypothyroidism | Hand movements only | 6/9 | Normal | Temporal superior quadrantanopia | Unchanged | Unchanged | Unchanged | Unchanged | Mild secondary hypothyroidism | Unchanged |
| 2 | 69 | F | 1 | HTN, RA | 6/5-1 | 6/9+2 | Normal | Inferior temporal quadrantanopia | Unchanged | Unchanged | Unchanged | Unchanged | Normal | Normal |
| 3 | 48 | F | 9 | T2DM, epilepsy | Hand movements only | 6/5 | Normal | Temporal superior quadrantanopia | Unchanged | Unchanged | Unchanged | Improved | Normal | Normal |
| 4 | 55 | M | 24 | Partial pan-hypopituitarism, epilepsy | Hand movements only | 6/5 | Near total loss | Temporal superior quadrantanopia | Improved to 6/9 | Unchanged | Improved visual fields | Unchanged | Partial hypopituitarism | Diabetes insipidus |
TABLE 1: Summary of Cases
Visual outcome
Of the 4 cases identified, 3 reported improvement in their visual field perception post-operatively. None of the cases identified reported any postoperative deterioration in their sight, with follow-up of these cases extending as far as 51 months in one case (range 1-51 months).
Pituitary outcome
Post-operative pituitary function remained unchanged in the majority of cases, with the exception of one patient with a pre-existing partial panhypopituitarism secondary to a prior debulking of their meningioma via craniotomy. This patient reported a deterioration of their Diabetes Insipidus symptoms following endoscopic surgery, requiring increased titration of Desmopressin therapy for a few days only. Of the two patients with preoperative endocrine dysfunction, neither showed an improvement in pituitary hormonal function following the procedure.
Discussion
In the context of meningioma resection, the endoscopic approach is being increasingly favoured. While craniotomy was the mainstay of treatment in the past, this approach was difficult for those meningiomas originating from the skull base [9]. These tumours primarily arise from the anterior cranial fossa, typically originating from the sphenoid wing, tuberculum sella or the olfactory groove and surgical access via craniotomy requires manipulation of regional neurovascular structures such as the optic apparatus as well as retraction of the frontal lobe, which can induce permanent behavioural changes [2,4,15,16-21]. Craniotomy also leaves patients at risk of bone-flap infections requiring re-operation, as well as potentially causing significant cosmetic deformity [11,22].
Although reports on using an endoscopic endonasal approach are becoming more commonplace, much of the focus is on the technical aspects of the surgical approach taken rather than the experience with differing pathologies [23]. Furthermore, these reports primarily describe the use of EET approaches to achieve total or as much resection as possible endoscopically (Simpson Grade I). The literature relating to the use of an endoscopic approach purely for the purpose of preserving vision is limited, despite this being one of the key treatment aims for management of anterior skull base meningiomas [24-26]. The cases and experience of these two neurosurgical centers serve to further add to the body of literature confirming the safety and reliability of an endoscopic approach in relieving optic nerve and chiasmal compression, even for those tumours that are so large that they have engulfed the surrounding neurovascular structures and pose too great a risk for complete resection. Our experience also suggests that despite large tumours compressing the optic apparatus with risk of permanent optic nerve atrophy, EET is a safe way to prevent further vision loss and that improvement in vision is possible in up to half of cases. However, given the limited number of cases identified and the retrospective nature of this analysis, generalisations on the use of EET for optic nerve and chiasm decompression must be limited.
Nevertheless, the endoscopic technique has been recognized as carrying low morbidity compared to craniotomy, as EET allows a direct approach to the meningioma without the need to manipulate the nervous structures. This avoids the risks of a traditional approach which can result in an ischaemic compressed optic apparatus when performing a bilateral optic canal decompression [20]. The minimal access approach also does not necessarily compromise degree of resection achievable as the inferior anteromedial trajectory allows for good access to these tumours which traditionally displace the optic apparatus posteriorly and superiorly [15,27]. This subtotal resection (Simpson Grade IV) is demonstrated in Figures 1-4 where a pre and post-operative coronal MR image demonstrates successful decompression of the optic nerve and chiasm.
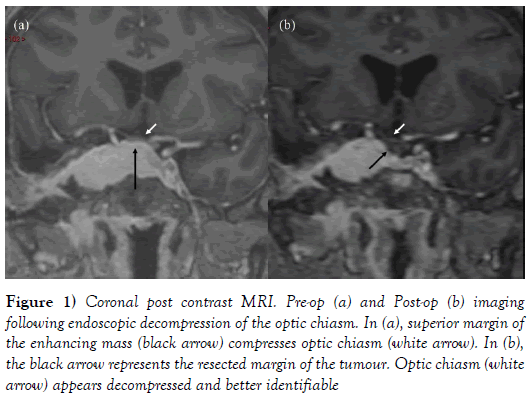
Figure 1: Coronal post contrast MRI. Pre-op (a) and Post-op (b) imaging following endoscopic decompression of the optic chiasm. In (a), superior margin of the enhancing mass (black arrow) compresses optic chiasm (white arrow). In (b), the black arrow represents the resected margin of the tumour. Optic chiasm (white arrow) appears decompressed and better identifiable
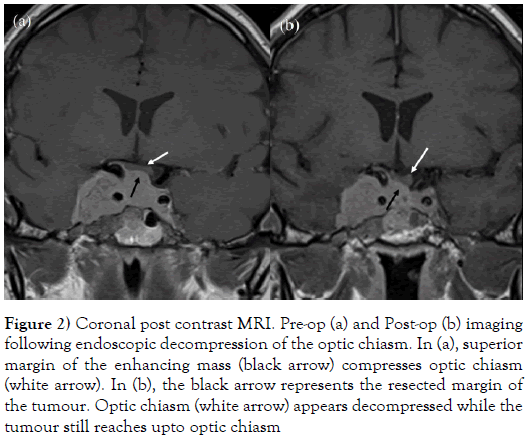
Figure 2: Coronal post contrast MRI. Pre-op (a) and Post-op (b) imaging following endoscopic decompression of the optic chiasm. In (a), superior margin of the enhancing mass (black arrow) compresses optic chiasm (white arrow). In (b), the black arrow represents the resected margin of the tumour. Optic chiasm (white arrow) appears decompressed while the tumour still reaches upto optic chiasm
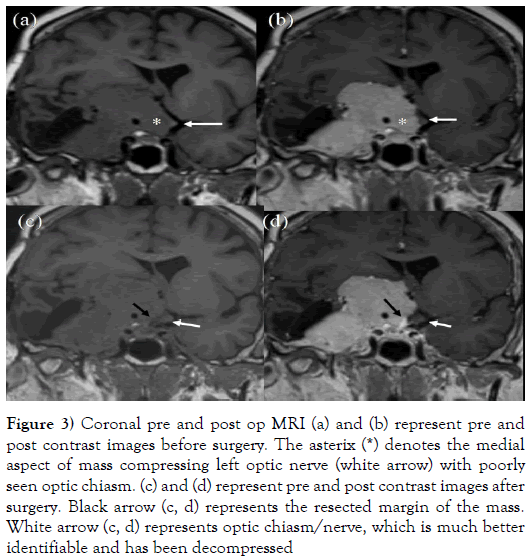
Figure 3: Coronal pre and post op MRI (a) and (b) represent pre and post contrast images before surgery. The asterix (*) denotes the medial aspect of mass compressing left optic nerve (white arrow) with poorly seen optic chiasm. (c) and (d) represent pre and post contrast images after surgery. Black arrow (c, d) represents the resected margin of the mass. White arrow (c, d) represents optic chiasm/nerve, which is much better identifiable and has been decompressed
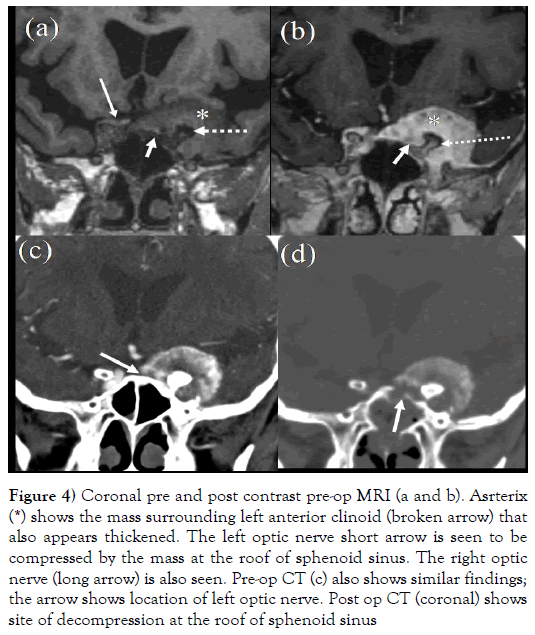
Figure 4: Coronal pre and post contrast pre-op MRI (a and b). Asrterix (*) shows the mass surrounding left anterior clinoid (broken arrow) that also appears thickened. The left optic nerve short arrow is seen to be compressed by the mass at the roof of sphenoid sinus. The right optic nerve (long arrow) is also seen. Pre-op CT (c) also shows similar findings; the arrow shows location of left optic nerve. Post op CT (coronal) shows site of decompression at the roof of sphenoid sinus
While the endoscopic approach does offer a lower complication profile, risks do remain, and this is demonstrated by the case of the patient whose pre-existing Diabetes Insipidus (DI) worsened, requiring increased doses of Desmopressin post-operatively. This was diagnosed with hourly urine input /output measurement and twice daily paired serum and urine sodium and osmolality measurements. DI is one of the relatively common and acknowledged complications of both trans-sphenoidal and traditional surgical approaches, with incidence rates of permanent post-operative DI being broadly similar between the two techniques [28,29]. Other key endoscopic complications to consider include cerebrospinal fluid leaks, bleeding (particularly in the context of resection of highly vascular tumours) and a potential worsening of vision [30,31]. However, in one study, rates of immediate DI post-operatively are lower for those treated endoscopically compared to craniotomy [29]. This may be explained by the endoscopic approach avoiding excessive manipulation of the posterior pituitary gland. This would theoretically result in less compression as well as less oedema of the pituitary stalk which are thought to be part of the pathogenesis of post-operative DI [30]. Furthermore, the endoscopic approach allows the surgeon to visualise early and avoid crossing key neurovascular structures, thereby reducing the risk of interrupting the blood supply to the posterior pituitary gland and it is notable that in this case, the patient’s DI and partial panhypopituitarism began following their first craniotomy for excision of their meningioma 3 years prior to the endoscopic procedure.
The success of this endoscopic approach is also highly dependent on good quality MRI imaging. Typically, in large skull base tumours, the optic chiasm, nerve and tract are significantly compressed and distorted, resulting in poor visualisation and difficulty in ascertaining the precise surgical target. Traditionally, MRI imaging of the brain and pituitary/skull base masses has relied on relatively thick sagittal and coronal T1-weighted images, both with and without contrast. Such images have limited usefulness in demonstrating the relationship of masses with the optic pathway. However, with recent improvements in technology, we can now perform volumetric MRI imaging that gives an excellent representation of the relationship between the optic pathways and masses, resulting in a better definition of the surgical target. This allows us to perform precisely guided minimally invasive surgery, ultimately resulting in better outcomes.
Conclusion
The experiences of these two centres add to the body of literature describing the use of an Endoscopic Endonasal Trans-Sphenoidal approach for optic chiasm and canal decompression. This suggests that this technique can be safely and reliably used in cases which are considered unsafe for complete resection via craniotomy; although further work is required to definitively demonstrate this. However, it is important to be mindful of the fact that this approach, while generally offering a lower risk profile than craniotomy, remains an advanced technique which can still result in neurosurgical complications or worsening of symptoms.
REFERENCES
- Wiemels J, Wrensch M, Claus EB. Epidemiology and etiology of meningioma. J Neurooncol 2010;99(3):307-14.
- Rubin G, Ben David U, Gornish M, et al. Meningiomas of the anterior cranial fossa floor. Review of 67 cases. Acta Neurochir 1994;129(1-2):26-30.
- Yang SY, Park CK, Park SH, et al. Atypical and anaplastic meningiomas: prognostic implications of clinicopathological features. J Neurol Neurosurg Psychiatry 2008;79(5):574-80.
- Komotar RJ, Starke RM, Raper DM, et al. Endoscopic endonasal versus open transcranial resection of anterior midline skull base meningiomas. World Neurosurg 2012;77(5-6):713-24.
- Chamberlain MC, Blumenthal DT. Intracranial meningiomas: diagnosis and treatment. Expert Rev Neurother 2004;4(4):641-8.
- Saraf S, McCarthy BJ, Villano JL. Update on meningiomas. Oncologist 2011;16(11):1604-13.
- Nakamura M, Struck M, Roser F, et al. Olfactory groove meningiomas: clinical outcome and recurrence rates after tumor removal through the frontolateral and bifrontal approach. Neurosurgery 2007;60(5):844-52.
- da Silva CE, de Freitas PEP. Large and giant skull base meningiomas: The role of radical surgical removal. Surgical Neurology International 2015;6:113.
- Chi JH, Parsa AT, Berger MS, et al. Extended bifrontal craniotomy for midline anterior fossa meningiomas: minimization of retraction-related edema and surgical outcomes. Neurosurgery 2006;59:ONS426-33.
- Rachinger W, Grau S, Tonn JC. Different microsurgical approaches to meningiomas of the anterior cranial base. Acta Neurochir 2010;152(6):931-9.
- Walcott BP, Redjal N, Coumans JV. Infection following operations on the central nervous system: deconstructing the myth of the sterile field. Neurosurg Focus 2012;33(5):E8.
- NHS Tayside - About Us 2018.
- NHS Grampian - About NHS Grampian 2018.
- Rivera-Serrano CM, Snyderman CH, Gardner P, et al. Nasoseptal "rescue" flap: a novel modification of the nasoseptal flap technique for pituitary surgery. Laryngoscope 2011;121(5):990-3.
- Kassam A, Snyderman CH, Mintz A, et al. Expanded endonasal approach: the rostrocaudal axis. Part I. Crista galli to the sella turcica. Neurosurg Focus 2005;19(1):E3.
- Padhye V, Naidoo Y, Alexander H, et al. Endoscopic endonasal resection of anterior skull base meningiomas. Otolaryngol Head Neck Surg 2012;147(3):575-82.
- Hayhurst C, Teo C. Tuberculum sella meningioma. Otolaryngol Clin North Am 2011;44(4):953-63.
- Dehdashti AR, Ganna A, Witterick I, et al. Expanded endoscopic endonasal approach for anterior cranial base and suprasellar lesions: indications and limitations. Neurosurgery 2009;64(4):677-87.
- Van Gompel JJ, Frank G, Pasquini E, et al. Expanded endonasal endoscopic resection of anterior fossa meningiomas: report of 13 cases and meta-analysis of the literature. Neurosurg Focus 2011;30(5):E15.
- Gardner PA, Kassam AB, Thomas A, et al. Endoscopic endonasal resection of anterior cranial base meningiomas. Neurosurgery 2008;63(1):36-52.
- de Divitiis E, Esposito F, Cappabianca P, et al. Endoscopic transnasal resection of anterior cranial fossa meningiomas. Neurosurg Focus 2008;25:E8.
- Delgado-Lopez PD, Martin-Velasco V, Castilla-Diez JM, et al. Preservation of bone flap after craniotomy infection. Neurocirugia 2009;20(6):124-31.
- Kong DS, Shin HJ, Kim HY, et al. Endoscopic optic canal decompression for compressive optic neuropathy. J Clin Neurosci 2011;18(2):1541-5.
- Attia M, Kandasamy J, Jakimovski D, et al. The importance and timing of optic canal exploration and decompression during endoscopic endonasal resection of tuberculum sella and planum sphenoidale meningiomas. Neurosurgery 2012;71(11):58-67.
- Lund VJ, Rose GE. Endoscopic transnasal orbital decompression for visual failure due to sphenoid wing meningioma. Eye 2006;20(10):1213-9.
- Pletcher SD, Sindwani R, Metson R. Endoscopic orbital and optic nerve decompression. Otolaryngol Clin North Am 2006;39(5):943-58.
- Berhouma M, Jacquesson T, Abouaf L, et al. Endoscopic endonasal optic nerve and orbital apex decompression for nontraumatic optic neuropathy: surgical nuances and review of the literature. Neurosurgical Focus 2014;37(4):E19.
- Schreckinger M, Szerlip N, Mittal S. Diabetes insipidus following resection of pituitary tumors. Clin Neurol Neurosurg 2013;115(2):121-6.
- Shah S, Har-El G. Diabetes insipidus after pituitary surgery: incidence after traditional versus endoscopic transsphenoidal approaches. Am J Rhinol 2001;15(6):377-9.
- Chowdhury T, Prabhakar H, Bithal PK, et al. Immediate postoperative complications in transsphenoidal pituitary surgery: A prospective study. Saudi J Anaesthesia 2014;8(3):335-41.
- Zimmer LA, Andaluz N. Incidence of epistaxis after endoscopic pituitary surgery: Proposed treatment algorithm. Ear Nose Throat J 2018;97(3):E44-e8.