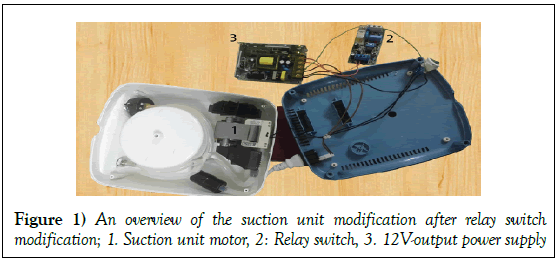
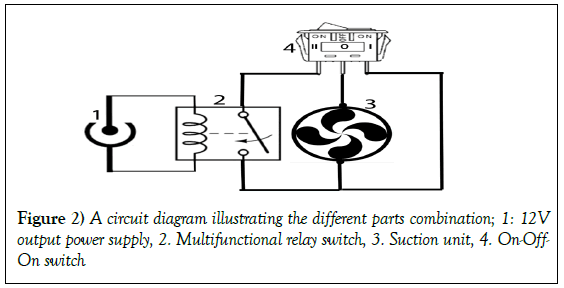
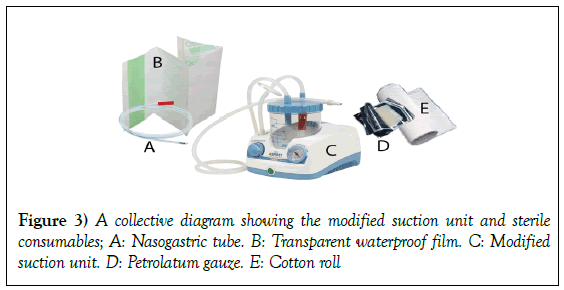
The use of economic negative-pressure wound therapy (NPWT) therapy machine and supplies in management of acute and chronic wounds: A report of 3 cases
Received: 22-Nov-2018 Accepted Date: Nov 22, 2018; Published: 06-Dec-2018
Citation: Abdelmoez A. The use of economic negative-pressure wound therapy (NPWT) therapy machine and supplies in management of acute and chronic wounds: A report of 3 cases. Surg Case Rep. 2018;2(2):39-41.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Negative-pressure wound therapy (NPWT) is known for improving a wide range of acute and chronic wounds. This technique has historic origins that go back to ancient times. Several theories were hypothesized around that technique of wound care including faster draining of the wound discharge, decreasing bacterial load of the wound, mechanical traction of wound edges by negative pressure and induction of wound surface hypoxia that encourages local angiogenesis. Using more economic machine, that was assembled by the author, and cheaper supplies on three different cases to test the efficacy of our method for wound care andhealing will be presented.
Keywords
NPWT; VAC therapy; Wound care; Innovation; Economic; Chronic wound
Introduction
The modern form of Negative-pressure wound therapy started to appear in the 1990s. However, the principle of using suction to heal wounds goes back to old history, precisely the Roman era [1]. During the Soviet- Afghan war (1979 – 1989), Russians developed the technique of using suction devices and foam to treat infected wounds [2]. Modern NPWT devices were developed by Argenta and Morykwas of Wake Forest University School of Medicine in the ninties [3]. Different theories and mechanisms were suggested to explain the healing potentials of negative pressure wound therapy technique.
Increase local wound blood flow
Morykwas proved that apply a negative pressure that equals -125 mmHg increased blood flow in swine wound models [4]. Lower pressures may increase blood flow in the long run [5]. Very low pressures (<-400 mmHg) have been shown to reduce the overall wound blood flow [4].
Mechanical wound contraction (Macrode formation)
Negative pressure applies contracting forces on the wound when applied in a sealed fashion, leading to mechanical reduction in the wound size [4,6,7].
Microde formation
Mechanical force generated by the negative pressure is transmitted to the cells through the extracellular matrix, leading to microde formation of cytoskeleton that stimulates cell proliferation by a process called mechano transduction [8]. These cellular changes lead to undulant wound surface due to negative suction at the foam–tissue interface [9].
Angiogenesis stimulation
Surface hypoxia induced by negative pressure and vascular endothelial growth factor (VEGF) expression along with mechanical forces were linked with formation of more physiological and straight blood vessels at the wound site [10].
Edema reduction
Infected wounds produce higher levels of exudate leading to edema. Negative pressure application removes excess wound fluid and reduces edema [11].
The same mechanism can explain low levels of anti-inflammatory mediators e.g. metalloproteinase, which breaks down adhesion proteins responsible for wound healing [12,13].
Decrease discharge and bacterial load of the wound
Wounds treated with NPWT showed a faster reduction in bacterial colonization [4], which can be explained by either decreasing wound discharge that discourages bacterial colonization or isolating the wound against outer environmental contamination [14,15].
Variables in applications
Pressure
Morykwas proved that -125 mmHg increased blood flow in swine wound models [4]. Lower pressures may increase blood flow in the long run [5]. Very low pressures (<-400 mmHg) have been shown to reduce the overall wound blood flow [4] (Figures 1-9).
Mode (continuous vs. intermittent)
Continuous mode was found to cause less pain [16,17]. However, intermittent mode was found to induce granulation faster [4] but cause more pain and can lead to exudate spillage into the wound during off period [16,17].
Filler material
Polyurethane foam can fit into deeper wounds [18], but antibacterial gauze caused less pain and fits into more superficial irregular surfaced wounds [19].
Frequency of dressing change
Varies from two to four days as time gaps between dressings [20].
Indications and contraindications
NPWT is indicated for temporary wound closure, wound preparation for final surgical closure, as an aid for successful graft take and adjunct therapy in wound reconstruction using dermal substitute [21]. On the other hand, it is contraindicated in wounds with very poor vascularity [14], deeper tissues infections e.g. osteomyelitis, malignancy, fistulas and undebrided wounds with necrotic tissues and eschars [22] (Figures 1-9).
Three patients were chosen to apply the technique of management.
Case Reports
NPWT was applied on three patients using the assembled device considering the following conditions:
Pressure: -125 mmHg, according to Morykwas recommendations [4].
Mode: Intermittent (5 minutes: ON and 3 minutes: OFF)
Frequency of dressing: Once every 48 hours sterile petrolatum gauze, sterile cotton roll, sterile nasogastric tube and sterile transparent waterproof film were used for dressing. The three cases can be briefly presented as following;
Case 1
A 52-year-old male, post road traffic accident, presented to our hospital around two weeks after the incident with nearly complete avulsion of the skin and subcutaneous tissues all around the left thigh. Wound was severely septic with heavy suppurative discharge. Patient was in a general septicemic bad condition with fever, high ESR and CRP, malaise and disturbed appetite. Wound swab for culture and sensitivity on admission showed heavy growth of Klebsiella pneumoniae, Acinetobacter baumannii and Proteus mirabilis.
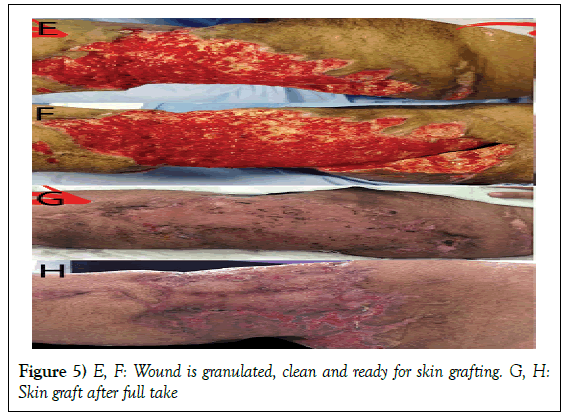
Management: Patient was taken directly to OR. Generous irrigation was done. Proximal and distal flaps were approximated using tension Ethibond sutures 5 to decrease the defect and eliminate any spaces. NPWT was applied in our method. Dressing was changed every two days. Healthy and clean granulation tissue was ready for split thickness skin grafting after 39 days.
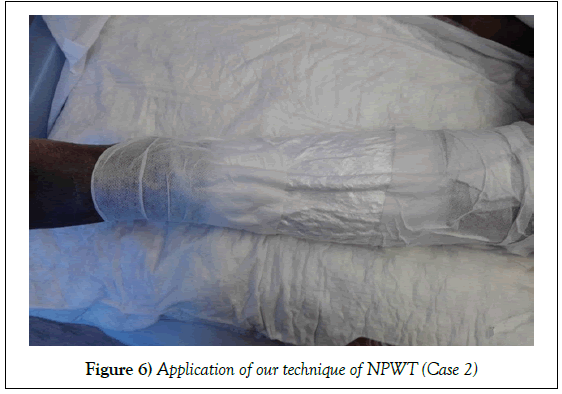
Case 2
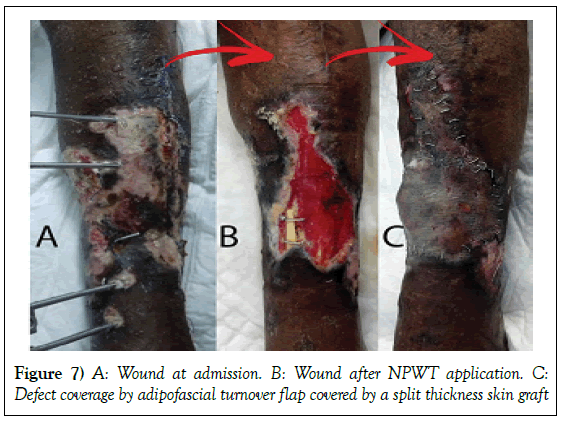
A 55-year-old female patient, post road traffic accident, presented to our hospital 12 days after the incident with right leg both bone open fracture (Gustilo IIIB) with a bony segmental loss from the right tibia, and skin and soft tissue loss at the same spot. Wound swab for culture and sensitivity on admission showed heavy growth of Klebsiella pneumoniae, Acinetobacter baumannii.
Management: Patient was shifted to OR, where she underwent wound debridement and fibular bone segment grafting. NPWT was applied in our technique. Wound was clean and granulated after 58 days. Exposed bone was covered by adipofascial turnover flap then a split thickness skin graft.
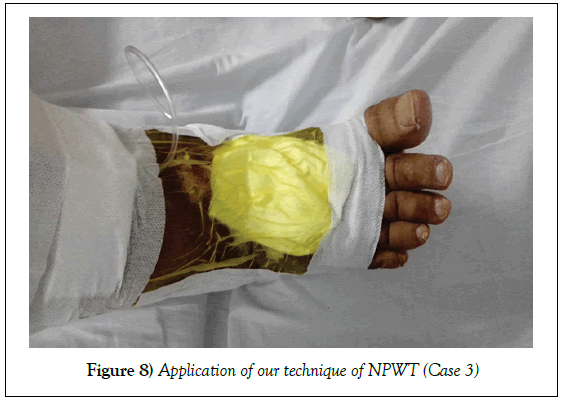
Case 3
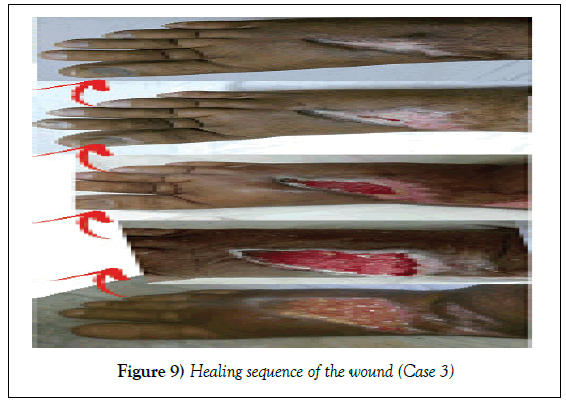
A 59-year--old male patient, underwent bariatric surgery four months ago. The surgery was complicated by DVT and deep, infected and highly discharging ulcer on the dorsum of the right foot. Patient presented to our hospital three months after unsuccessful trials of wound care methods. Wound swab for culture and sensitivity on admission showed heavy growth of Klebsiella pneumonia.
Management: Wound was irrigated. NPWT was applied in our technique. Wound was completely healed without any further surgical procedures in 66 days.
Discussion and Conclusion
Application of the NPWT principle of treatment using an economic modified suction unit and cheaper affordable consumables gives significantly positive results of improvement in different kinds of wounds as shown in the case report.
REFERENCES
- Harris T. Poisoned wounds. The Medical Examiner. 1838;1: 250-4.
- Otterbourg K. The bloody patent battle over a healing machine. Fortune Management. CNN Money,2012.
- John HD. Negative pressure wound therapy. Health Professions Institute. 2008:17-8.
- Morykwas MJ, Argenta LC, Shelton-Brown EI, et al. Vacuum-assisted closure: a new method for wound control and treatment: animal studies and basic foundation. Ann Plast Surg. 1997;38(6):553-62.
- Timmers MS, Le Cessie S, Banwell P, et al. The effects of varying degrees of pressure delivered by negative-pressure wound therapy on skin perfusion. Ann Plast Surg. 2005;55(6):665-71.
- Schintler MV. Negative pressure therapy: Theory and practice. Diabetes Metab Res Rev. 2012;28:72-7.
- Lavery LA, Boulton AJ, Niezgoda JA, et al. A comparison of diabetic foot ulcer outcomes using negative pressure wound therapy versus historical standard of care. Int Wound J. 2007;4(2):103-13.
- Scherer SS, Pietramaggiori G, Mathews JC, et al. The mechanism of action of vacuum assisted closure device. Plast Reconst Surg 2008;122(3):786-99.
- Huang C, Leavitt T, Bayer LR, et al. Effect of negative pressure wound therapy on wound healing. Curr Probl Surg 2014;51:301-31.
- Erba P, Ogawa R, Ackermann M, et al. Angiogenesis in wounds treated by micro-deformational wound therapy. Ann Surg 2011;253(2):402-9
- Lu X, Chen S, Li X. The experimental study of the effects of vacuum-assisted closure on edema and vessel permeability of the wound. Chin J Clin Rehab.
- Buttenschoen K, Fleischmann W, Haupt U, et al. The influence of vacuum-assisted closure on inflammatory tissue reactions in the postoperative course of ankle fractures. Foot Ankle Surg. 2001;7(3):165-73.
- Gustafsson R, Johnsson P, Algotsson L, et al. Vacuum assisted closure therapy guided by C-reactive protein level in patients with deep sternal wound infection. J Thorac Cardiovasc Surg. 2002;123(5):895-900.
- Vig S, Dowsett C, Berg L, et al. Evidence-based recommendations for the use of negative pressure wound therapy in chronic wounds: steps towards an international consensus. J Tissue Viability. 2011;20:1-18.
- Chan SY, Wong KL, Lim JX, et al. The role of Renasys-GO in the treatment of diabetic lower limb ulcers: A case series. Diabet Foot Ankle. 2014;5(1):24718.
- Borgquist O, Ingemansson R, Malmsjo M. Individualizing the negative pressure wound therapy for optimal wound healing: a focused review of literature. Ostomy Wound Manage 2011;57(4):44-54.
- Malmsjo M, Gustafsson L, Lindstedt S, et al. The effects of variable, intermittent and continuous negative pressure wound therapy, using foam or gauze on wound contraction, granulation tissue formation and ingrowth into wound filler. Eplasty 2012;11:42-54.
- Yang CC, Chang DS, Webb LX. Vacuum assisted closure for fasciotomy wounds following compartment syndrome of leg. J Surg Orthop Adv 2006;15(1):19-23
- Hurd T, Chadwick P, Cote J, et al. Impact of gauze based NPWT on the patient and nursing experience in the treatment of challenging wounds. Int Wound J 2010;7(6):448-455.
- Hinck D, Franke A, Gatzka F. Use of vacuum-assisted closure negative pressure wound therapy in combat-related injuries—literature review. Mil Med 2010;175(3):173-181.
- Sanjay M, Singh BP. Adv Wound Care. 2016;5(9):379-389.
- Hasan M, Teo Y, Nather RA. Negative-pressure wound therapy for management of diabetic foot wounds: a review of the mechanism of olfaction, clinical applications, and recent developments. Diabetic Foot & Ankle. 2015;6(1):27618.