The utility of cerebrospinal fluid biomarkers of AlzheimerŌĆÖs disease and the APOE genotype in the characterisation of mild cognitive impairment and prodromal AlzheimerŌĆÖs disease
Received: 06-Oct-2018 Accepted Date: Nov 16, 2018; Published: 23-Nov-2018
Citation: Garcia-Estevez DA. The utility of cerebrospinal fluid biomarkers of alzheimer├āŲÆ├éŲÆ├āŌĆÜ├é┬ó├āŲÆ├éŌĆÜ├āŌĆÜ├éŌé¼├āŲÆ├éŌĆÜ├āŌĆÜ├éŌäós disease and the APOE genotype in the characterisation of mild cognitive impairment and prodromal alzheimer├āŲÆ├éŲÆ├āŌĆÜ├é┬ó├āŲÆ├éŌĆÜ├āŌĆÜ├éŌé¼├āŲÆ├éŌĆÜ├āŌĆÜ├éŌäós disease. J Neurol Clin Neurosci. 2018;2(4):05-07.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Introduction: The use of biomarkers (β-amyloid peptide and Tau protein) in the cerebrospinal fluid (CSF) is useful to identify patients with mild cognitive impairment (MCI) due to Alzheimer’s disease (AD). Other variables, such as a family history of dementia, medial temporal atrophy (MTA) and the ApoE genotype can also help characterize patients with MCI due to AD.
Aim: To identify, using CSF biomarkers for AD in a series of patients with amnestic MCI, those with true prodromal AD and the variables that characterize them.
Subjects and Methods: Forty-one patients with amnestic MCI were studied and evaluated using the memory alteration test (MAT). Biomarkers for AD were determined in the CSF, and the patients classified as having amnestic MCI due to AD if the Hulstaert index was ≤ 1.0 and levels of phosphorylated tau protein ≥ 61 pg/mL. MTA was assessed by MRI using Scheltens visual rating scale. All patients were found to have the ApoE genotype.
Results: Thirty of the patients were classified as having MCI due to AD. Compared with cases that were not due to AD, no significant differences were found with regard to age, sex, family history of dementia, MAT score or MTA measured with the visual rating scale. The group with MCI due to AD had a higher prevalence of the ε4 allele (63.3% vs. 18.2%, p=0.012).
Conclusions: Amnestic MCI can be classified as prodromal AD based on CSF biomarkers and the ApoE genotype, but not based on the assessment of MTA using a visual rating scale.
Introduction
There is extensive literature characterizing mild cognitive impairment (MCI) as a multi-aetiological entity that affects one or several cognitive domains and with an annual rate of progression to clinical dementia of around 10%, although the concept encompasses patients in whom the clinical course does not involve clinical progression of impairment, and others who even show cognitive improvement. The miscellany that is MCI affects a wide range of patients with different disorders including systemic diseases, drug-induced cognitive impairment and psychiatric diseases (mainly depression and anxiety), which could explain the variable clinical course [1- 3]. Although the diagnosis of MCI is clinical, in this scenario, the detection of both neuroimaging and biochemical biomarkers in the cerebrospinal fluid (CSF), which provide a measure of neuronal damage (hippocampal atrophy on magnetic resonance imaging [MRI] and CSF Tau protein) and cerebral amyloid deposition (Pittsburgh compound B [PiB] on PET scan and CSF β-amyloid peptide) have allowed the characterization of MCI as due to Alzheimer’s disease (AD/MCI ) if there is evidence that both pathogenic pathways (β amyloid and Tau protein) are altered in a given patient; so that we appear to be faced with true prodromal Alzheimer’s disease (AD) here [4-6]. This is the stage of disease where the utmost must be done to prevent disease progression by resorting to pharmacological approaches such as cognitive stimulation and promoting clinical trials.
The apparently complex diagnostic procedure can be carried out outside clinics specializing in dementia and tertiary care hospitals; this article thus describes the actions taken in our general neurology unit in a regional hospital, in patients with amnestic MCI using CSF biomarkers of AD to diagnose the prodromal phase of AD, which we believe to constitute an improvement in the quality of care for patients with cognitive impairment.
Subjects and Methods
We studied patients with amnestic MCI in a general neurology unit who were diagnosed according to the Petersen criteria [7] and the Spanish Neurology Society criteria for the clinical diagnosis of MCI [8]. The patients were referred from Primary Care due to cognitive symptoms such as shortterm memory loss. The first visit involved clinical history-taking to determine the presence of hippocampal amnesia that did not improve with semantic clues and demonstrate that the patient could carry out instrumental activities of daily living independently, and to rule out secondary causes for the cognitive deficits, such as medications that interfere with cognition (tricyclic antidepressants, benzodiazepines, opiaceous drugs), systemic disease (hepatic, pulmonary or renal chronic diseases) or the presence of persistent depressive disorder. Depression and/or anxiety were discarded through a specific medical interview. They then underwent the Mini Mental State Examination (MMSE) where they had to score ≥26/30 (adjusted for educational level), and episodic memory was assessed using the Memory Alteration Test (MAT) [9]. The patients thus selected underwent brain MRI to assess hippocampal and medial temporal lobe atrophy. The degree of atrophy was quantified by the Radiology Department of our hospital using the Scheltens visual rating scale [10] which classifies it into 5 levels (from 0 to 4) depending on the scores assigned to the width of the Choroid fissure, the radial width of the temporal horn and the length of the hippocampus (Table 1). For the statistical analysis, due to small size of the groups, they were grouped into two categories, one for levels 0-2 (normal-minimal temporal atrophy) and the other for levels 3-4 (severe temporal atrophy).
| Score | Width of the Choroid fissure | Radial width of the temporal horn | Length of the hippocampus |
|---|---|---|---|
| 0 | Normal | Normal | Normal |
| 1 | ↑ | Normal | Normal |
| 2 | ↑↑ | ↑ | ↓ |
| 3 | ↑↑↑ | ↑↑ | ↓↓ |
| 4 | ↑↑↑ | ↑↑↑ | ↓↓↓ |
| Adapted from Scheltens et al. JNNP 1992, 55: 967-72. | |||
Table 1: Scheltens visual rating scale for medial temporal atrophy.
A second visit was scheduled at 3 months to check for the persistence of memory deficit and to tell the patient and caregiver about the possibility of diagnosing AD in the prodromal phase by performing a lumbar puncture and analysing CSF biomarkers, for which written informed consent was requested. In addition, the apolipoprotein E (ApoE) genotype was determined in all patients as a risk marker for the development of AD. Lumbar puncture was performed between 9:00 and 11:00 am in the lateral decubitus position. Three samples of 1 mL of CSF were extracted in polypropylene tubes for the determination of β-amyloid peptide 1-42, total Tau protein and phosphorylated Tau protein at position 181 (p-Tau). The samples were frozen at -80º until testing.
The Hulstaert index (HI) was calculated according to the following formula:
HI = (β-amyloid peptide)/240+1.18 x total Tau [11],
and the index AD-CSF index (p-Tau) proposed by Molinuevo et al. [12]:
AD-CSF index (p-Tau) = ((1.200- β amyloid peptide) / 1.075) + (pTau - 15) / 285.
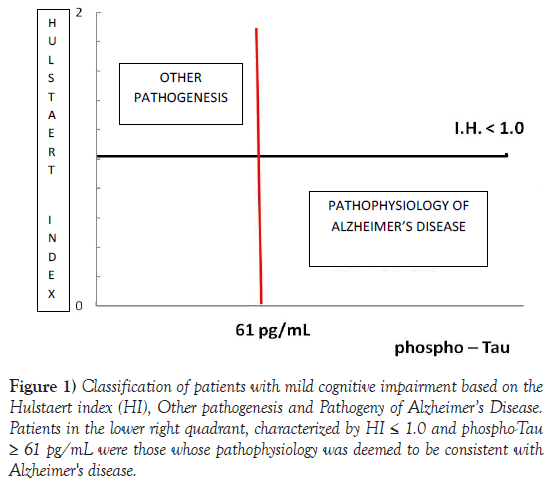
The patients were classified under amnestic MCI due to AD if they had a HI ≤1.0 and p-Tau ≥ 61 pg mL (Figure 1).
Figure 1) Classification of patients with mild cognitive impairment based on the Hulstaert index (HI), Other pathogenesis and Pathogeny of Alzheimer’s Disease. Patients in the lower right quadrant, characterized by HI = 1.0 and phospho-Tau = 61 pg/mL were those whose pathophysiology was deemed to be consistent with Alzheimer's disease.
The statistical analysis was carried out using the SPSS® software platform. Student’s test was used for between-group comparisons of mean basal parameter values, following verification of normality and subsequent logarithmic or square-root transformation when necessary. The results are expressed as median and range.
Results
Thirty of the 41 patients were classified as having MCI due to AD. The differences between the two groups are shown in Table 2. There were no differences between groups in terms of age, sex or family history of dementia. Neither was any differences observed between the two groups in terms of the MAT score.
| MCI-no AD | MCI-AD | P | |
|---|---|---|---|
| N | 11 | 30 | |
| Age (a) | 74 (65–84) | 73 (60-83) | NS |
| Sex (F/M) | 4-July | 18-December | NS |
| FH of dementia (%) | 36.4 | 53.3 | NS |
| MAT score | 27 (17–44) | 24 (16–37) | NS |
| β-amyloid (pg/mL) | 1,000 (683-1,810) | 719 (125-972) | <0.001 |
| Total Tau (pg/mL) | 264 (187–322) | 552 (208–1,200) | <0.001 |
| p-Tau (pg/mL) | 46 (25-57) | 88 (61–160) | <0.001 |
| Hulstaert index | 1.85 (1.08–3.039) | 0.66 (0.26–1.00) | <0.001 |
| AD-CSF index (p-Tau) | 0.31 (0.06–0.57) | 0.75 (0.61-1.68) | <0.001 |
| MTA [0-2/3-4] | 6-May | 18-December | NS |
| ApoE ε4 allele (%) | 18.2 | 63.3 | 0.012 |
| ACEI treatment (%) | 18.2 | 86.7 | <0.001 |
FH: family history. MAT: Memory Alteration Test, MTA: Medial Temporal Atrophy assessed with the Scheltens visual rating scale. ACEI: Acetylcholinesterase Inhibitors.
Table 2: Demographic, clinical and paraclinical variables of the groups with MCI.
All CSF biomarkers were significantly altered (decreased β-amyloid, and elevation of total Tau protein and p-Tau) in patients with MCI-AD. Conspicuously, although with a median value of 719 pg/mL, levels of β-amyloid peptide were high, HI was significantly decreased to a range typical of AD. Within the same principle, patients with MCI-AD showed significant elevation of the AD-CSF-index (pTau).
There were no significant differences between the two groups in terms of the medial temporal atrophy score determined with the Scheltens visual rating scale, i.e., the number of patients with grade 3-4 atrophy was no higher in the MCI-AD group than in the MCI-no AD group. When the sample was dichotomised by degree of atrophy, no significant differences were observed in MAT score or HI.
The MCI-AD group showed a higher prevalence of the ApoE ε4 allele (18.2% vs 63.3%, p=0.012).
Discussion
The clinical-investigative approach to patients with cognitive impairment differs substantially between specialized dementia consultations and general neurology clinics where the clinical approach and the practical application of recommendations from neurologist specializing in dementia prevail. One example in this context is the use of both MRI and CSF biomarkers for the characterization of amnestic MCI as prodromal AD. This approach is likely to provide added value to the quality of care of patients with cognitive impairment.
Although MCI is an entity with multiple aetiologies and an annual rate of progression to clinical dementia of 7-10%, there are currently no approved pharmacological treatments for this progressive phase of dementia. The clinical and therapeutic benefit of using biomarkers in MCI to determine whether it is due to AD is tremendous, as it allows identifying AD in its prodromal phase and justifies the initiation of treatment with acetylcholinesterase inhibitors (ACEI) in these patients in order to try to slow down disease progression.
Cognitive tests commonly used to assess episodic memory in patients with cognitive impairment were of no use for identifying amnestic MCI when the physiopathogenesis was due to AD. Our study focused on the application of MAT, and we found no differences between groups and no associations with CSF biomarkers or medial temporal atrophy on MRI.
MRI functions as a biomarker of MCI-AD, allowing identifying neuronal damage, atrophy of the cerebral cortex and parenchyma, and the presence of ischemic lesions that may contribute to the progression of cognitive impairment. In general neurology clinics, there is limited access to neuroimaging studies that use three-dimensional software to assess hippocampal volume, which is decreased in amnestic MCI and AD, but not in multi-domain MCI [13]. However, the detection of medial temporal lobe atrophy using the easy-to-apply Scheltens visual rating scale has proved useful for the diagnosis of AD [14], and MTA has been correlated with progression to dementia in MCI [15]. In our daily clinical practice, we use the Scheltens scale to assess the degree of hippocampal and medial temporal lobe atrophy. However, in our series, it proved of little use to identify patients with MCIAD, in that more severe atrophy was not associated with a decreased MAT score or Hulstaert index. Another approach to assessing neuronal damage which we used in our study relies on CSF biomarkers, specifically elevated levels of total and phosphorylated Tau protein, the latter adding specificity to the diagnosis of AD.
We use imaging techniques such as PiB-PET, which is currently restricted to a few tertiary care/research centres to assess the physiopathological process of cerebral amyloid deposition. Though more accessible, the determination of CSF levels of β-amyloid 1-42 also involves more invasive methods. This biomarker is decreased in patients with AD, which is attributed to its deposition in cerebral amyloid plaques. It is well known that CSF biomarkers are also altered in patients with MCI who progress to dementia during follow-up [16]. In our series, although the behaviour of biomarkers showed the involvement of the two pathogenic pathways involved in AD (amyloid and Tau protein), what was most interesting from a clinical viewpoint was the index that correlates β-amyloid peptide with total Tau protein, known as the Hulstaert index, and p-Tau levels, since both allow to classify amnestic MCI as prodromal AD. In this regard, previous international [16] and national [17] publications have shown that the combination of biomarkers is a highly accurate and specific predictor of progression from MCI to dementia.
Patients with MCI-AD tended to have a more extensive family history of dementia, but the difference was not statistically significant. However, the ApoE genotype was discriminative, in that the ε4 allele was more prevalent in the MCI-AD group. This supports the diagnostic probability of presenting with AD based on the increased relative risk conferred by the presence of this allele in the context of dementia [18] and highlights the predictive value of MCI progression to AD [19].
The diagnostic procedure allowed starting ACEI treatment (which is not approved in MCI) early in about 90% of the patients, in combination with cognitive stimulation therapy. However, current evidence does not support treatment with donepezil, rivastigmine or galantamine to prevent the progression of MCI to clinical dementia [20], although it should be noted that patients in these studies were selected solely on the basis of clinical criteria and not based on biomarkers. Part of the current therapeutic approach focuses on treating aggravating factors of cognitive decline such as depression and cardiovascular risk factors, especially hypertension, which requires more focus and emphasis in patients with MCI-AD. In addition, while donepezil could be effective in the cohort of patients with MCI associated with depression [21], this aspect was not adequately assessed in our study, since we did not make strict enough use of scales validated for the diagnosis of depression to allow conclusions to be drawn in this regard.
Our study, which was conducted under conditions of daily clinical practice, allows concluding the following: 1) amnestic MCI can be classified as prodromal AD based on the use of AD biomarkers in the CSF and the presence of at least one ApoE ε4 allele. 2) the assessment of medial temporal atrophy with a visual rating scale does not allow to differentiate patients with MCI-AD from those who do not have a pathogenesis characteristic of AD, and 3) the identification of prodromal AD allows early initiation of specific ACEI treatment.
REFERENCES
- Petersen RC, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Arch Neurol. 2001,58:1985-92.
- Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183-94.
- Alberca R. Mild cognitive impairment in daily clinical practice of the neurologist and primary care physician. In Alzheimer’s Disease. Rey Pérez A, LLeó Bisa A. editors. Editorial Médica Panamericana (Madrid). 2010;pp:1-19.
- Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Age and Alzheimer’s Association Workgroup. Alzheimers Dement. 2011;7:270-9.
- Dubois B, Feldman HH, Jacova C, et al. Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007;6:734-46.
- Dubois B, Feldman HH, Jacova C, et al. Revising the definition of Alzheimer’s disease: A new lexicon. Lancet Neurol. 2010;9:1118-27.
- Petersen RC, Smith GE, Waring SC, et al. Mild cognitive impairment. Clinical characterization and outcome. Arch Neurol. 1999;56:303-8.
- Robles A, Del Ser T, Alom J, et al. Proposal of criteria for clinical diagnosis of mild cognitive impairment, dementia and Alzheimer’s disease. Neurología. 2002;17:17-32.
- Rami L, Molinuevo JL, Sanchez-Valle R, et al. Screening for amnestic mild cognitive impairment and early Alzheimer’s disease with M@T (Memory Alteration Test) in the primary care population. Int J Geriatr Psychiatr. 2007;24:274-9.
- Scheltens Ph, Leys D, Barkhof F, et al. Atrophy of medial lobes on MRI in “probable” Alzheimer’s disease and normal ageing: diagnostic value and neuropsychological correlates. J Neurol Neurosurg Psychiatry. 1992;55:967-72.
- Hulstaert F, Blennow K, Ivanoiu A, et al. Improved discrimination of AD patients using beta-amyloid (1-42) and tau levels in CSF. Neurology 1999;52:1555-62.
- Molinuevo JL, Gispert JD, Pujol J, et al. A new approach to the Alzheimer’s disease diagnosis with biomarkers: description of the AD-CSF-Index. Rev Neurol. 2012;54:513-22.
- Becker JT, Davis SW, Hayashi KM, et al. Three-dimensional patterns of hippocampal atrophy in mild cognitive impairment. Arch Neurol. 2 006;63:97-101.
- Galton CJ, Gomez-Anson B, Antoun N, et al. Temporal lobe rating scale: Application to Alzheimer’s disease and frontotemporal dementia. J Neurol Neurosurg Psychiatry. 2001;70:165-73.
- Korf ESC, Wahlund LO, Visser PJ, et al. Medial temporal lobe atrophy on MRI predicts dementia in patients with mild cognitive impairment. Neurol. 2004:63:94-100.
- Hansson O, Zetterberg H, Buchhave P, et al. Association between CSF biomarkers and incipient Alzheimer’s disease in patients with mild cognitive impairment: a follow-up study. Lancet Neurol. 2006;5:228-34.
- Monge-Argiles JA, Munoz-Ruiz C, Pampliega-Perez A, et al. Biomarkers of Alzheimer’s disease in the cerebrospinal fluid of Spanish patients with mild cognitive impairment. Neurochem Res. 2011;36:986-93.
- Farrer LA, Cupples LA, Haines HL, et al. Effects of age, sex and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease: a major gene with semi-dominant inheritance. Mol Psychiatry. 2011;16:903-7.
- Petersen RC, Smith GE, Ivnik RJ, et al. Apolipoprotein E status as a predictor of the development of Alzheimer’s disease in memory-impaired individuals. JAMA. 1995;273:1274-8.
- Cooper C, Li R, Lyketsos C, et al. Treatment for mild cognitive impairment: systematic review. B J Psych. 2013;203:255-64.
- Pelton GH, Harper OL, Tabert MH, et al. Randomized double-blind placebo-controlled donepezil augmentation in antidepressant-treated elderly patients with depression and cognitive impairment: a pilot study. Int J Geriatr Psychiatry. 2008;23:670-6.
Keywords
Mild cognitive impairment; Prodromal Alzheimer’s disease; β-amyloid peptide; Tau protein; ApoE genotype; Hulstaert’s index; AD-CSF index.