Time to stop using immunosuppressive agents in chronic glomerulonephritis
Received: 12-Jan-2023, Manuscript No. PULJMBR-23-6046; Editor assigned: 16-Jan-2023, Pre QC No. PULJMBR-23-6046 (PQ); Accepted Date: Jan 25, 2023; Reviewed: 20-Jan-2023 QC No. PULJMBR-23-6046 (Q); Revised: 22-Jan-2023, Manuscript No. PULJMBR-23-6046 (R); Published: 27-Jan-2023, DOI: 10.37532/puljmbr.2023.6(1).19-23
Citation: Sharaf El Din UAA, Salem MM, Abdulazim DO. Time to stop using immunosuppressive agents in chronic glomerulonephritis. J Mic Bio Rep. 2023;6(1):19-23.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Chronic glomerulonephritis is the third most important cause of end stage renal disease worldwide. Most of the patients suffering different types of glomerulonephritis are prescribed different immunosuppressive agents aiming at complete or partial remission in order to stop or muffle the progression of chronic kidney disease. Although the initial injury in case of glomerulonephritis is the glomerulus, the best predictor of renal survival is the amount of tubulointerstitial fibrosis. Recent trials have proved the efficacy of the anti-diabetic sodium glucose cotransporters-2 inhibitors in non-diabetic chronic kidney disease to significantly decrease the renal end points. Chronic glomerulonephritis represented a considerable bulk of nondiabetic cases recruited in these studies. This success should stimulate clinical Nephrologists to consider these agents in the newly diagnosed cases suffering increased urine protein excretion instead of immunosppressive treatment. In this review we will try to elucidate the value of these agents in management of patients presenting with different types of chronic glomerulonephritis
Keywords
Chronic glomerulonephritis; Focal segmental glomerulosclerosis; FSGS; IgA nephropathy; Membranoproliferative glomerulonephritis
Introduction
Glomerular Diseases (GDs), whether primary or secondary, occur as a sequence of systemic autoimmune diseases, infections, drugs, or malignancy. GDs can affect individuals of all ages. Examples of these diseases include primary Focal Segmental Glomerulosclerosis (FSGS), IgA Nephropathy (IgAN), Membranous Glomerulonephritis (MGN), Membranoproliferative Glomerulonephritis (MPGN), Lupus Nephritis (LN), ANCA-Associated Vasculitis (AAV), and Minimal Change Nephrotic Syndrome (MCN). In most kidney failure registries, GDs account for 20%– 25% of End-Stage Renal Disease (ESRD) cases. In children, teenagers, and young adults, GDs are the most common cause of irreversible kidney damage [1]. Most Glomerulonephritis (GN) patients have been prescribed steroids and other Immunosuppressive (IS) agents aiming at stopping or delaying the progression to ESRD. The use of IS agents in patients suffering from GN is associated with a high risk of adverse effects that affect patients’ morbidity and mortality. These adverse effects include opportunistic infections, bone disease, hyperglycemia, obesity, systemic hypertension, disturbed mentality, gastroduodenal ulcerations, cataracts, and cardiovascular disease. The risk of opportunistic infection might be exaggerated in GN patients because of the urinary loss of immunoglobulins [2]. The rate of infection in GN kept on IS agents might exceed five folds the rate of those cases that are exempted from this treatment [3]. These adverse effects have imposed a careful balance of risk and benefits when using such agents.
Two years ago, the results of the Dapagliflozin (Dapa) in Patients with Chronic Kidney Disease (DAPA-CKD) study demonstrated for the first time the value of Dapa in non-diabetic kidney disease patients. DAPA reduced the primary composite outcome (sustained decline in the eGFR by>50%, ESRD, and renal or cardiovascular death) by 39% while the incidence of adverse events and serious adverse events was similar in the dapa and placebo groups [4]. A few weeks ago, the results of the EMPA-kidney study evolved. After of median of two years of follow-up of 6609 patients suffering Diabetic Kidney Disease (DKD) or non-diabetic Chronic Kidney Disease (CKD) that were randomized to treatment with 10 mg empagliflozin (EMPA) or placebo, progression of kidney disease or death from cardiovascular causes occurred in 13.1% of the EMPA group and in 16.9% of the placebo group (P<0.001). Results were consistent among patients with or without diabetes and regardless of the Estimated Glomerular Filtration Rate (eGFR). Again, the rates of serious adverse events were similar in EMPA and placebo groups [5]. The impact of different sodium glucose cotransporters-2 inhibitors (SGLT2Is) on Urine Albumin Excretion in patients (UAE) suffering from DKD was demonstrated in EMPA-REG, CANVAS, and DECLARE-TIMI 58. However, being a surrogate marker, the more recent CREDENCE, DAPA-CKD, and EMPA- kidney studies were more concerned with the clinical endpoints, namely the rate of deterioration in kidney function, need for dialysis, hospitalization, and mortality. In a case report of a male patient suffering from FSGS, the addition of DAPA to his treatment regimen significantly reduced UAE [6]. In this review, we will illustrate the different mechanisms that enable SGLT2Is to replace the IS treatment in different types of GN.
Literature Review
Role of the Proximal Convoluted Tubules (PCT)
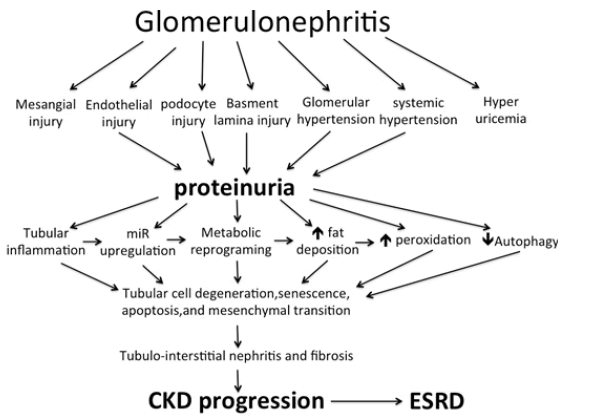
Recent studies have shifted the attention from the glomerulus and the renal interstitium towards the PCT as the primary sensor and effector in the progression of CKD in most renal diseases including chronic GN. PCT epithelium has 2 eminent criteria that render their cells vulnerable to injury. These cells are packed with mitochondria and are dependent on oxidative phosphorylation as a source of energy. These two criteria make these cells liable to degeneration and death on exposure to ischemic, hypoxic, oxidative, metabolic, and/or obstructive insult [7,8]. Excess protein leakage through injured glomeruli with consequently increased protein absorption by PCT leads to tubular degeneration. Excess tubular protein absorption is associated with increased activity of heme oxygenase and Monocyte Chemoattractant Protein (MCP1) [9]. Damaged epithelial cells fail to produce an adequate amount of Vascular Endothelial Growth Factor (VEGF) necessary for the maintenance of intact peritubular capillaries. Rarefaction of peritubular capillaries results in ischemia of PCT with consequent perturbation of tubular damage [7]. Initially, PCT responses to GN include metabolic changes, impaired autophagy, de-differentiation, and cell cycle changes as an adaptive response. However, these responses can become maladaptive and promote Tubulointerstitial Fibrosis (TIF). TIF is the hallmark of CKD and the best predictor of ESRD [8]. Growth factors produced by damaged PCT cells include the Transforming Growth Factor Beta (TGFβ), Wnt proteins, and platelet-derived growth factor β (PDGF-β). These growth factors stimulate myofibroblast formation, proliferation, and Extracellular Matrix (ECM) production [9].
Albumin handling within the kidney
The presence of Albuminuria together with eGFR constitutes the bases of the diagnosis of CKD. Proteinuria is a strong and independent predictor for CKD progression and renal outcome. The glomerular filtration barrier is composed of a 3-layer structure (the endothelium, the glomerular basement lamina, and the podocytes). Increased intraglomerular hydrostatic pressure or damaged glomerular filtration barrier can elicit proteinuria. Albumin is filtered by the glomeruli and reabsorbed by the PCT through receptormediated endocytosis. Internalization by endocytosis is followed by transport into lysosomes for degradation. The multi-ligand receptors megalin and cubilin are responsible for tubular Albumin uptake In normal adults, Low Molecular Weight (LMW) proteins are freely filtered at the glomerulus. The filtered LMW proteins are almost completely actively reabsorbed by the PCT [10]. About 9.6 grams of LMW proteins and 3 grams of Albumin are daily reabsorbed by the renal tubules [11]. In normal personnel, renal tubules absorb almost all the Albumin that leaks through the glomerular membrane [10]. After the delivery of Albumin to lysosomes, dissociation between megalin and Albumin occurs to enable megalin to deliver more Albumin molecules from the tubular lumen. This dissociation is activated by lysosomal acidification by H+ ATPase. This ATPase is inhibited by Angiotensin II (ATII). Angiotensin II within renal tissue increases in response to hyperglycemia or increased free oxygen species within the cytoplasm. Thus, inhibition of ATII by Renin-Angiotensin System (RAS) blockers increases megalin availability and enhances tubular Albumin absorption [12]. In the case of renal disease, proteinuria indicates either leakage through the glomerular filtration barrier (when urine Albumin prevails) or tubular dysfunction if LMW protein is the major constituent [13]. Glomerular proteinuria usually indicates an active primary glomerular disease, adaptive hyperfiltration, or glomerular scarring [14]. The mechanisms underlying glomerular disease include inflammatory cell infiltration, glomerular cell proliferation, and podocytopathy. Podocyte dysfunction is a sequence of nephrin or podocin defects.
Within PCT cells, Albumin- ligand complex induces the expression of inflammatory and fibrogenic mediators. TGF-β, which may be induced by Albumin exposure, may also act in a feedback mechanism increasing Albumin filtration and at the same time inhibiting megalin- and cubilin-mediated Albumin endocytosis, leading to increased Albuminuria [15-17]. Multiple pathways are involved, including induction of chemokine expression within PCT cells, cytokines, and complement activation, which lead to interstitial nephritis and fibrosis and consequent loss of kidney function [17]. The higher the proteinuria, the more likely the progress of renal insufficiency [18-20]. However, the selectivity of proteinuria has a significant impact. When proteinuria is mainly composed of Albumin, the renal damage might be milder than in the case of non-selective proteinuria where the ultrafiltrate contains potentially nephrotoxic proteins, such as complement proteins, immunoglobulins, and growth factors [13,17]. However, it has been demonstrated that the byproducts of Albumin breakdown within PCT cells induce dendritic cells to induce inflammation [21].
Metabolic reprogramming in chronic GN
The kidney is the second organ in the rate of oxygen consumption after the heart. The renal tubules spend a tremendous amount of Adenosine Triphosphate (ATP) during the elimination of different waste products and reabsorption of most of the filtered water and valuable solutes [22]. While the Distal Convoluted Tubules (DCT) depend on anaerobic glycolysis as the main source of ATP due to the low oxygen tension, the PCT relies mainly on Fatty Acid Oxidation (FAO) and gluconeogenesis [23]. Normal energy metabolism guarantees the maintenance of healthy structure and function of the kidney. Metabolic reprogramming was initially described in cancer cells that metabolically adapt to the changes in environmental conditions to meet the energy consumption and proliferation requirements. Recent studies have demonstrated that renal cells are also capable of metabolic reprogramming after kidney injury. This metabolic reprogramming plays a pivotal role in the progression and prognosis of chronic GN. Disturbed glucose and lipid metabolism are always observed in patients progressing to ESRD [22]. In response to inflammation, a shift from oxidative phosphorylation to aerobic glycolysis (glycolysis in presence of adequate oxygen) is observed in glucose metabolism. This switch to aerobic glycolysis reduces the expression of acetyl-CoA, with the consequent increase in the expression of fibrotic genes in tubular epithelium, podocytes, and fibroblasts [23]. Accumulating unused fatty acids are stored as a triglyceride. Excess cell fat induces epithelial degeneration [22]. These metabolic changes promote epithelium mesenchymal transition [24]. In this process, epithelial cells transform into fibroblasts that produce an extracellular matrix aiming at the healing of tissues injured by inflammation and stabilizing the injured tubules [25]. Although the kidney can recover from acute inflammation, persistent or severe inflammation can cause fibrosis. Once fibrosis starts, it is extremely difficult to stop or reverse, and this process gradually leads to complete loss of kidney function [26]. In addition to injured parenchyma, infiltrating inflammatory cells are an additional source of the cytokines responsible for metabolic reprogramming with the consequent renal fibrosis. TGFβ, the most eminent of these cytokines, downregulates Peroxisome ProliferatorActivated Receptors (PPARs) that have a role in FAO and are responsible for the metabolic shift of glucose metabolism from oxidative phosphorylation to glycolysis despite adequate oxygen supply (aerobic glycolysis) [27-29]. Aerobic glycolysis is a faster source of Adenosine Triphosphate (ATP), redirects carbon to the production of pentoses, nucleic acid synthesis, and formation of non-essential amino acids such as glycine, which constitutes 35% of all the amino acids within collagen [30-32]. Lactic acid produced by glycolysis increase cytoplasm acidity that stimulates TGFβ activity and thus a vicious cycle ensues [33]. These metabolic changes that occur as a consequence of inflammation globally simulate the Warburg effect that was initially described in cancer cells and are responsible for the disturbed cell proliferation, extracellular matrix production, suppression of autophagy, and apoptosis that are observed in chronic kidney disease [34-36]. After the initial activation of fibroblasts by TGFβ released as a sequence of inflammation, they continue to act autonomously independent of inflammation [37]. It is worth mentioning that the TGFβ effect is mediated by Hypoxia-Inducible Factor-1α (HIF-1α) [38].
In support of the role of metabolic reprogramming on renal fibrosis, Zhao et al. demonstrated that stimulation of FAO succeeded to suppress Extracellular Matrix (ECM) accumulation and fibrosis [39].
Impact of metabolic reprogramming on interstitial inflammation
The damage induced in renal tubular epithelial cells by metabolic reprogramming allows the recruitment of inflammatory cells to the renal interstitium. Consequently, these inflammatory cells produce numerous proinflammatory and pro-fibrotic cytokines. Macrophages and T lymphocytes constitute the majority of recruited inflammatory cells. The M1 macrophage subtype prevails in the case of activated aerobic glycolysis instead of the M2 subtype that prevails during normal oxidative phosphorylation [40]. T-cell infiltration occurs during kidney injury and may directly increase the inflammatory response, and the pro-fibrotic phenotype of macrophages [41]. Metabolic reprogramming is closely related to the activation of T cells [42].
MicroRNAs and glomerulonephritis
MicroRNAs (MiRs) are small non-coding ribonucleic acid molecules that have a pivotal role in post-transcriptional gene regulation. MiRs have been studied in pre-clinical and clinical studies of the pathogenesis of glomerulonephritis and tubulointerstitial fibrosis [43]. MiRs interfere with protein synthesis run by the messenger RNA molecules transcription or can degrade the different RNA subtypes involved in protein synthesis. In this manner, miRs modulate the expression of different proteins implicated in cell differentiation, proliferation, and apoptosis. Thousands of miRs have been discovered in humans. Different miRs are found excessively expressed in different organs in different cells in different diseases. In one report, glomeruli obtained from cases suffering FSGS showed increased expression of miR-193a, cases having ANCA-associated GN showed increased expression of miR-155, while cases suffering Ig A nephropathy and lupus nephritis have increased expression of miR-148b, and miR-148a-3p respectively [44]. Other studies showed elevated urinary miR-155 in IgA nephropathy [45]. miR-155 is implicated in renal fibrosis even in ureteric obstruction [46]. MiR-21 upregulation within the PCT aggravates metabolic reprogramming by silencing PPAR-mediated FAO and the genes that inhibit reactive oxygen species generation within the mitochondria [47].
SGLT2Is and GN
Most of the time, the administration of RAS blockers and IS treatment fail to prevent the progression of chronic GN to ESRD. In the DAPACKD study, 104 patients with biopsy-proven FSGS having eGFR 25 mL/ min/1.73m2 –75 mL/min/1.73m2 , UAE 200 mg/gram –5000 mg/gram, and were prescribed a stable dose of Angiotensin-Converting Enzyme Inhibitors (ACEi) or Angiotensin Receptor Blockers (ARB) for at least 4 weeks before enrolment into the trial were randomized to either DAPA 10 mg or placebo. The primary outcome occurred less frequently and the rate of eGFR decline was lower in the DAPA group [48]. The number of IgAN patients recruited for the DAPA-CKD study is the largest of all known IgA trials. DAPA-CKD included 271 IgAN patients. These patients showed an unprecedented 71% reduction in the primary outcome [49]. This result dictates that SGLT2Is may be more beneficial than immunosuppression in IgAN patients.
A trial of DAPA 10 mg in 17 Chinese LN cases (one male and 16 females, with disease duration 7.3 years ± 5 years, UAE 0.5 grams/day -3 grams/day) that were administered 10 mg DAPA in addition to their standard of care treatment (mean prednisolone dose 16.3 mg/day ± 7.1 mg/day, 6 were on>20 mg/day, 6 were on mycophenolate mofetil, 3 on cyclophosphamide, and 2 on tacrolimus). DAPA was tried for 6 months. DAPA had an acceptable safety profile despite being administered on top of other IS treatment. Improvement in the slope of GFR was observed in patients having baseline eGFR<90mL/ min/1.73 m2 [50]. Patients with AAV and systemic lupus erythematosus have a significantly increased risk for cardiovascular morbidity and mortality, with both inflammation and long-term use of immunosuppressants contributing to this risk. In patients with AAV with kidney involvement, CKD is a strong cardiovascular risk factor. Efforts to prevent CKD progression with SGLT2Is may have a positive effect on patient outcomes. In these patients, like other forms of GN, specific targeted therapy combined with SGLT2Is would gain a maximal therapeutic effect [51]. So far, no studies have been performed on AAV. SGLT2 was detected in podocytes of mice treated with Bovine Serum Albumin (BSA). SGLT2 is upregulated in podocytes after BSA injections. Podocytes were directly targeted by SGLT2Is that maintain their actin cytoskeleton architecture and limit their damage [52]. Pleiotropic effects of SGLT2Is on different renal cells besides PCT cells have been suggested by recent studies. SGLT2Is may act directly on endothelial cells by modification of the adhesion molecules’ effects and by reducing intracellular inflammatory cytokines and reactive oxygen species. They additionally inhibit inflammatory and profibrotic effects within PCT cells. Some reports suggest direct protective effects on mesangial cells as well [53].
Renal effects of SGLT2Is in GN
Sodium-Glucose Co-Transporters (SGLTs) are members of the mammalian solute carrier family SLC5. This SLC5 family is responsible for the active, sodium-driven transport of different sugars, anions, vitamins, and short-chain fatty acids in different tissues [54]. Renal SGLTs are located within the PCT cell as SGLT2 in S1 and S2 segments and are responsible for the absorption of 90% of glucose delivered in the ultrafiltrate, while SGLT1 within the S3 segment absorbs the remaining 10%. SGLT2Is inhibit the reabsorption of glucose and sodium in PCT. Although the primary target of SGLT2Is as renoprotective agents is mediated through their tubular action, they have many pleiotropic cellular actions that ameliorate the downstream injury from the original glomerular damages. The potential renoprotective mechanisms include promoting diuresis and natriuresis through the inhibition of SGLTs and the renal Sodium-Hydrogen Exchanger3 (NHE3) isoform. This effect of SGLT2Is is mainly mediated through NHE3. In contrast to wild mice, Empa failed to induce natriuresis in normoglycemic NHE3 knock-out mice [55]. Increasing sodium delivery to DCT with a consequent increase in adenosine production normalizing tubuloglomerular feedback and lowering glomerular hyperfiltration [56]. Decreasing oxygen consumption by PCT thus decreases the induction of Hypoxia-Inducible Factor-1α (HIF-1 α) with a consequent decrease in inflammation and fibrogenesis [57]. HIF-1 α and AngiopoietinLike 4 expression are upregulated within FSGS kidneys. SGLT2Is inhibit the expression of these two agents [58]. Increasing hypoxia in DCT thus increases induction of HIF-1 β with consequently increased erythropoiesis, decreased inflammation and fibrogenesis [59]. By reducing glucose uptake by PCT, even in normoglycemic patients, SGLT2Is augment AMPK/SIRT1 signaling and stimulate autophagy, thereby decreasing the accelerated damage of renal tubular cells as well as podocytes [60-62]. Stimulation of AMPK leads to inhibition of the mammalian target of rapamycin (mTORC1) which is responsible for tubular and podocyte damage, proteinuria, and fibrosis [63]. Although the existence of SGLT2s within human podocytes is still tentative, amelioration of proteinuria on using DAPA was associated with decreased effacement of foot processes. Despite the controversy about the presence SGLTs in renal endothelial cells, accumulating evidence suggests that SGLT2Is likely act directly on these cells, via modulating the adhesion molecules and reducing inflammatory cytokines [53]. By inhibiting PCT glucose absorption, SGLT2Is decrease intracellular fructose and thus decrease intracellular Uric Acid (UA) synthesis [64]. Intracellular UA stimulates Nicotinamide Adenine Dinucleotide Phosphate (NADPH) oxidase that increases the production of Reactive Oxygen Species (ROS). Increased intracellular UA and ROS activate Nalp3 which induces the activation of caspase 1 [65]. Glucose wasting induced by SGLT2Is causes calorie depletion and simulates fasting or nutrient deprivation state. Consequently, FAO is stimulated and lipid accumulation is inhibited. In addition, SGLT2Is suppress the production of hexosamine. Suppression of hexosamine and lipid synthesis inhibit Protein Kinase C (PKC) and TGF β activation [56]. Suppression of PKC and TGFβ decreases the chance of Epithelial Mesenchyme Transition (EMT), Endothelial Mesenchyme Transition (EnMT), interstitial inflammation, and fibrosis. By improving miR signature, SGLT2Is prevent suppression of FAO and overproduction of ROS [47]. With the loss of some nephrons due to tubular damage, the remaining nephrons compensate by glomerular hyperfiltration. This glomerular hyperfiltration causes podocyte shear stress that ultimately leads to podocyte detachment, proteinuria, and secondary focal segmental glomerulosclerosis that progressively proceeds to diffuse global glomerulosclerosis [66,67]. The metabolic overload imposed over PCT promotes irreversible loss of PCT cells via the urine and ultimately promotes tubule atrophy. These two events lead to the progression of CKD and the shortening of the kidney lifespan [68]. By protecting against glomerular detachment and tubular cell damage, SGLT2Is save the kidneys of chronic GN against CKD progression and ESRD (Figure 1).
Figure 1: Mechanism of chronic kidney progression in patients suffering chronic glomerulonephritis. miR= micro RNA; CKD= Chronic Kidney Disease; ESRD= End Stage Renal Disease. SGLT2Is combat different renal cells injury, glomerular hypertension, systemic hypertension, hyperuricemia, tubular cell inflammation, miR upregulation, metabolic reprogramming, fatty infiltration, free oxygen radical injury, and iduce autophagy
Adverse effects of SGLT2Is
In the DAPA-CKD trial, DAPA was well tolerated. The rate of serious adverse events was similar in patients treated with DAPA and placebo groups. There was no reported case of severe hypoglycemia in nondiabetic patients with CKD [4]. Similar findings were reported in the EMPA- kidney study [5]. In these 2 studies, patients kept on steroids and other IS drugs were excluded for fear of increased incidence of infection. However, many small observation studies did not encounter an increased incidence of adverse events, including infections, in patients treated with a combination of SGLT2Is and IS drugs [69,70].
Discussion and Conclusion
Despite the recent advances in pharmacological interventions, acute and chronic glomerular diseases continue to result in substantial morbidity and mortality. Steroids and other IS agents frequently used in the management of chronic GN carry the potential risks of endangering the quality of life of these patients and may haste mortality. The striking renoprotective effects of SGLT2Is in patients suffering from DKD have encouraged investigators to look for similar benefits in non-diabetic CKD. These agents proved a considerable impact, not only on renal survival but also on patient survival making the use of these agents a mandate in these cases. In 2021, Kidney Disease Improved Global Outcome (KDIGO) released updated guidelines for the management of GN. These guidelines suggest using ACEi/ARBs as the first-line supportive treatment in patients with GN, hypertension, and proteinuria (>1 gram/day) with up-titration depending on blood pressure response. By the time of issuing these guidelines, the results of DAPA-CKD and EMPA-kidney studies were not yet disclosed. Today, SGLT2Is should be added to the maximum tolerable dose of ACEi/ARBs to gain the maximum therapeutic benefit. Nephrology practice became different after SGLT2Is. The introduction of SGLT2Is should be advised to all cases suffering GN. This action would significantly reduce the need for steroids and other IS agents. SGLT2Is are cheaper and safer than steroids and most of the other IS treatments. According to the available data, severe cases that might still need the introduction of IS treatment can be safely prescribed these agents on top of ACEi/ARBs and SGLT2Is. Expectedly, the doses of such IS agents would be needed would be much less than that used previously. It seems that Nephrology practice has dramatically changed after SGLT2Is. We still need further studies that look after the impact of the addition of fenofibrate to SGLT2Is and the maximum tolerable dose of ACEi/ARB on the outcome of GN in different ages.
References
- Rovin BH, Adler SG, Barratt J, et al. KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney international. 2021;100(4): S1-276.
- Jefferson JA. Complications of immunosuppression in glomerular disease. Clin J Am Soc Nephrol.: CJASN. 2018;13(8):1264.
- Fardet L, Petersen I, Nazareth I. Common infections in patients prescribed systemic glucocorticoids in primary care: a population-based cohort study. PLoS medicine. 2016;13(5): e1002024.
- Heerspink HJ, Stefansson BV, Correa-Rotter R, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med.2020;383(15):1436-46.
- EMPA-Kidney Collaborative Group. Empagliflozin in patients with chronic kidney disease. N Engl J Med. 2022.
- Sjuls S, Jensen U, Littmann K, et al. Effective cholesterol lowering after myocardial infarction in patients with nephrotic syndrome may require a multi-pharmacological approach: a case report. Eur Heart J-Case Rep. 2021;5(5): ytab151.
- Chevalier RL. The proximal tubule is the primary target of injury and progression of kidney disease: role of the glomerulotubular junction. Am J Physiol-Ren Physiol. 2016;311(1): F145-61.
- Gewin LS. Renal fibrosis: primacy of the proximal tubule. Matrix Biology. 2018; 68:248-62.
- Motoyoshi Y, Matsusaka T, Saito A, et al. Megalin contributes to the early injury of proximal tubule cells during nonselective proteinuria. Kidney international. 2008;74(10):1262-9.
- Tojo A, Endou H. Intrarenal handling of proteins in rats using fractional micropuncture technique. Am J Physiol-Ren Physiol. 1992;263(4): F601-6.
- Tojo A, Kinugasa S. Mechanisms of glomerular Albumin filtration and tubular reabsorption. Int j nephrol. 2012.
- Andersen S, Blouch K, Bialek J, et al. Glomerular permselectivity in early stages of overt diabetic nephropathy. Kidney international. 2000;58(5):2129-37.
- Kopp JB, Anders HJ, Susztak K, et al. Podocytopathies. Nat Rev Dis Primers. 2020 ;6(1):1-24. [Google Scholar]
- Gorriz JL, Martinez-Castelao A. Proteinuria: detection and role in native renal disease progression. Transplantation reviews. 2012;26(1):3-13.
- Drumm K, Bauer B, Freudinger R, et al. Albumin induces NF-κB expression in human proximal tubule-derived cells (IHKE-1). Cell Physiol Biochem 2002;12(4):187-96.
- Cravedi P, Remuzzi G. Pathophysiology of proteinuria and its value as an outcome measure in chronic kidney disease. Br j clin pharmacol. 2013;76(4):516-23.
- Ruggenenti P, Perna A, Mosconi L, et al. Proteinuria predicts end-stage renal failure in non-diabetic chronic nephropathies. Kidney International Supplement. 1997.
- Peterson JC, Adler S, Burkart JM, et al. Blood pressure control, proteinuria, and the progression of renal disease: the Modification of Diet in Renal Disease Study. Ann intern med. 1995;123(10):754-62.
- Wright Jr JT, Bakris G, Greene T, et al. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. Jama. 2002;288(19):2421-31.
- Macconi D, Chiabrando C, Schiarea S, et al. Proteasomal processing of Albumin by renal dendritic cells generates antigenic peptides. J Am Soc Nephrol.: JASN. 2009;20(1):123.
- Bhargava P, Schnellmann RG. Mitochondrial energetics in the kidney. Nat Rev Nephrol. 2017;13(10):629-46.
- Cargill K, Sims-Lucas S. Metabolic requirements of the nephron. Pediatric Nephrology. 2020;35(1):1-8.
- Li Y, Sha Z, Peng H. Metabolic Reprogramming in Kidney Diseases: Evidence and Therapeutic Opportunities. Int j nephrol. 2021.
- Irifuku T, Doi S, Sasaki K, et al. Inhibition of H3K9 histone methyltransferase G9a attenuates renal fibrosis and retains klotho expression. Kidney International. 2016;89(1):147-57.
- Walther TC, Chung J, Farese Jr RV. Lipid droplet biogenesis. Annu rev cell dev biol. 2017; 33:491.
- Kang HM, Ahn SH, Choi P, et al. Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nature medicine. 2015;21(1):37-46.
- Kaissling B, LeHir M, Kriz W. Renal epithelial injury and fibrosis. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease. 2013;1832(7):931-9.
- Hewitson TD, Smith ER. A Metabolic Reprogramming of Glycolysis and Glutamine Metabolism Is a Requisite for Renal Fibrogenesis—Why and How?. Frontiers in Physiology. 2021:288.
- Lakshmi SP, Reddy AT, Reddy RC. Transforming growth factor β suppresses peroxisome proliferator-activated receptor γ expression via both SMAD binding and novel TGF-β inhibitory elements. Biochemical Journal. 2017;474(9):1531-46.
- Meng XM, Nikolic-Paterson DJ, Lan HY. TGF-β: the master regulator of fibrosis. Nat Rev Nephrol. 2016;12(6):325-38.
- Para R, Romero F, George G, et al. Metabolic reprogramming as a driver of fibroblast activation in pulmonaryfibrosis. Am j med sci. 2019;357(5):394-8.
- Nigdelioglu R, Hamanaka RB, Meliton AY, et al. Transforming growth factor (TGF)-β promotes de novo serine synthesis for collagen production. J Biol Chem. 2016;291(53):27239-51.
- Bernard K, Logsdon NJ, Ravi S, et al. Metabolic reprogramming is required for myofibroblast contractility and differentiation. J Biol Chem. 2015 ;290(42):25427-38.
- Selvarajah B, Azuelos I, Plate M, et al. mTORC1 amplifies the ATF4-dependent de novo serine-glycine pathway to supply glycine during TGF-β1–induced collagen biosynthesis. Science signaling. 2019;12(582): eaav3048.
- Chen Z, Liu M, Li L, et al. Involvement of the Warburg effect in non‐tumor diseases processes. J cell physiol. 2018;233(4):2839-49.
- Tan VP, Miyamoto S. HK2/hexokinase-II integrates glycolysis and autophagy to confer cellular protection. Autophagy. 2015;11(6):963-4.
- Zhu X, Jiang L, Long M, et al. Metabolic Reprogramming and Renal Fibrosis. Frontiers in Medicine. 2021;8.
- Calvier L, Chouvarine P, Legchenko E, et al. PPARγ links BMP2 and TGFβ1 pathways in vascular smooth muscle cells, regulating cell proliferation and glucose metabolism. Cell metabolism. 2017;25(5):1118-34.
- Zhao X, Psarianos P, Ghoraie LS, et al. Metabolic regulation of dermal fibroblasts contributes to skin extracellular matrix homeostasis and fibrosis. Nature metabolism. 2019;1(1):147-57.
- Wei T, Gao J, Huang C, et al. SIRT3 (sirtuin-3) prevents Ang II (angiotensin II)–induced macrophage metabolic switch improving perivascular adipose tissue function. Arteriosclerosis, Thrombosis, and Vascular Biology. 2021;41(2):714-30.
[Google Scholar] [Cross Ref]
- Nikolic-Paterson DJ. CD4+ T cells: a potential player in renal fibrosis. Kidney international. 2010;78(4):333-5. [Google Scholar]
- Ravi S, Mitchell T, Kramer PA, et al. Mitochondria in monocytes and macrophages-implications for translational and basic research. int j biochem.cell biol. 2014; 53:202-7.
- Peters LJ, Floege J, Biessen EA, et al. MicroRNAs in chronic kidney disease: four candidates for clinical application. Int j mol sci. 2020;21(18):6547.
- Trionfini P, Benigni A. MicroRNAs as master regulators of glomerular function in health and disease. J Am Soc Nephrol.: JASN. 2017;28(6):1686.
- Wang G, Kwan BC, Lai FM, et al. Elevated levels of miR-146a and miR-155 in kidney biopsy and urine from patients with IgA nephropathy. Disease markers. 2011;30(4):171-9.
- Zhang W, Li X, Tang Y, et al. miR-155-5p implicates in the pathogenesis of renal fibrosis via targeting SOCS1 and SOCS6. Oxidative med cell longev. 2020.
- Gomez IG, Grafals M, Portilla D, et al. MicroRNAs as potential therapeutic targets in kidney disease. J Formos Med Assoc. 2013;112(5):237-43.
- Das NA, Carpenter AJ, Belenchia A, et al. Empagliflozin reduces high glucose-induced oxidative stress and miR-21-dependent TRAF3IP2 induction and RECK suppression, and inhibits human renal proximal tubular epithelial cell migration and epithelial-to-mesenchymal transition. Cellular signalling. 2020; 68:109506.
- Anders HJ, Peired AJ, Romagnani P. SGLT2 inhibition requires reconsideration of fundamental paradigms in chronic kidney disease,‘diabetic nephropathy’, IgA nephropathy and podocytopathies with FSGS lesions. Nephrology Dialysis Transplantation. 2022 ;37(9):1609-15.
- Wang H, Li T, Sun F, et al. Safety and efficacy of the SGLT2 inhibitor dapagliflozin in patients with systemic lupus erythematosus: a phase I/II trial. RMD open. 2022 ;8(2): e002686.
- Saemann M, Kronbichler A. Call for action in ANCA-associated vasculitis and lupus nephritis: promises and challenges of SGLT-2 inhibitors. Ann Rheum Dis. 2022;81(5):614-7.
- Cassis P, Locatelli M, Cerullo D, et al. SGLT2 inhibitor dapagliflozin limits podocyte damage in proteinuric nondiabetic nephropathy. JCI insight. 2018;3(15).
- Miyata KN, Zhang SL, Chan JS. The rationale and evidence for SGLT2 inhibitors as a treatment for nondiabetic glomerular disease. Glomerular Diseases. 2021;1(1):21-33.
- Gyimesi G, Pujol-Gimenez J, Kanai Y, et al. Sodium-coupled glucose transport, the SLC5 family, and therapeutically relevant inhibitors: from molecular discovery to clinical application. Pflug Arch-Eur J Physiol. 2020;472(9):1177-206.
- Onishi A, Fu Y, Patel R, et al. A role for tubular Na+/H+ exchanger NHE3 in the natriuretic effect of the SGLT2 inhibitor empagliflozin. Am J Physiol-Ren Physiol. 2020;319(4): F712-28.
- El Din UA, Salem MM, Abdulazim DO. Sodium-glucose cotransporter 2 inhibitors as the first universal treatment of chronic kidney disease. nefrologia. 2022;42(4):390-403.
- Packer M. Mechanisms leading to differential hypoxia-inducible factor signaling in the diabetic kidney: modulation by SGLT2 inhibitors and hypoxia mimetics. Am J Kidney Dis. 2021;77(2):280-6.
- Basu D, Huggins LA, Scerbo D, et al. Mechanism of increased LDL (low-density lipoprotein) and decreased triglycerides with SGLT2 (sodium-glucose cotransporter 2) inhibition. Arteriosclerosis, thrombosis, and vascular biology. 2018;38(9):2207-16.
- Packer M. Mutual antagonism of hypoxia-inducible factor isoforms in cardiac, vascular, and renal disorders. Basic to Translational Science. 2020;5(9):961-8.
- DeFronzo RA, Hompesch M, Kasichayanula S, et al. Characterization of renal glucose reabsorption in response to dapagliflozin in healthy subjects and subjects with type 2 diabetes. Diabetes care. 2013;36(10):3169-76.
- Packer M. Role of impaired nutrient and oxygen deprivation signaling and deficient autophagic flux in diabetic CKD development: implications for understanding the effects of sodium-glucose cotransporter 2-inhibitors. J Am Soc Nephrol: JASN. 2020;31(5):907.
- Zhou J, Zhu J, Yu SJ, et al. Sodium-glucose co-transporter-2 (SGLT-2) inhibition reduces glucose uptake to induce breast cancer cell growth arrest through AMPK/mTOR pathway. Biomedicine & Pharmacotherapy. 2020; 132:110821.
- Kogot-Levin A, Hinden L, Riahi Y, et al. Proximal tubule mTORC1 is a central player in the pathophysiology of diabetic nephropathy and its correction by SGLT2 inhibitors. Cell reports. 2020;32(4):107954.
- List JF, Whaley JM. Glucose dynamics and mechanistic implications of SGLT2 inhibitors in animals and humans. Kidney International. 2011;79: S20-7.
- Nicholas SA, Bubnov VV, Yasinska IM, et al. Involvement of xanthine oxidase and hypoxia-inducible factor 1 in Toll-like receptor 7/8-mediated activation of caspase 1 and interleukin-1β. Cell Mol Life Sci. 2011;68(1):151-8.
- Kriz W, Lemley KV. A potential role for mechanical forces in the detachment of podocytes and the progression of CKD. J Am Soc Nephrol: JASN. 2015;26(2):258.
- Hodgin JB, Bitzer M, Wickman L, et al. Glomerular aging and focal global glomerulosclerosis: a podometric perspective. J Am Soc Nephrol: JASN. 2015 ;26(12):3162.
- Romagnani P, Remuzzi G, Glassock R, et al. Chronic kidney disease. Nature reviews Disease primers. 2017;3(1):1-24.
- Song CC, Brown A, Winstead R, et al. Early initiation of sodium‐glucose linked transporter inhibitors (SGLT‐2i) and associated metabolic and electrolyte outcomes in diabetic kidney transplant recipients. Endocrinology, Diabetes & Metabolism. 2021;4(2): e00185.
- AlKindi F, Al-Omary HL, Hussain Q, et al. Outcomes of SGLT2 inhibitors use in diabetic renal transplant patients. In Transplantation proceedings 2020; 52(1):175-178.