Variations observed of the internal iliac artery and their possible clinical implications
2 NYIT College of Osteopathic Medicine, Northern Blvd, Old Westbury, New York 11568, USA, Email: bbeatty@nyit.edu
Received: 31-Mar-2023, Manuscript No. ijav-23-6293; Editor assigned: 03-Apr-2023, Pre QC No. ijav-23-6293 (PQ); Accepted Date: Apr 21, 2023; Reviewed: 17-Apr-2023 QC No. ijav-23-6293; Revised: 21-Apr-2023, Manuscript No. ijav-23-6293 (R); Published: 28-Apr-2023, DOI: 10.37532/1308-4038.16(4).252
Citation: Khan S, Beatty BL. Variations Observed of the Internal Iliac Artery and their Possible Clinical Implications. Int J Anat Var. 2023;16(4):278-280.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Understanding the variation of the internal iliac artery (IIA) is of interest to the medical community because vascular compromise of this region can lead to pathologies like IIA occlusive disease, and lower extremity arterial disease. Anatomic variation can lead to confusion in surgeries such as embolization and angioplasty or can lead to lingering symptoms after surgery. We studied variations in the internal iliac artery, which contains the inferior gluteal, internal pudendal, middle rectal, obturator, umbilical, superior vesical, inferior vesical, uterine, superior gluteal, lumbosacral, and iliolumbar arteries. 25 cadavers were studied and, for each individual, branching patterns of 10 arteries from both the anterior and posterior divisions of the internal iliac artery were observed. Our most interesting observations were of a muscular artery entering the psoas muscle, because it has never been reported before. We found this branch in 13/25 of the cadavers studied, where the majority of them were coming off the superior gluteal artery. Another interesting observation was of the uterine artery, where 4 of them originated from the common trunk of the superior vesical and umbilical arteries. We also found significant variation in the obturator artery, but this has been previously well studied. These studies suggest that compromise of vessels that are not normally considered high risk for pathologies such as gait issues or severe bladder dysfunction should be assessed properly in the treatment plan of a patient. Significant variation exists in the pelvic region, and if not appropriately evaluated, can lead to unforeseen end organ damage.
Keywords
Anatomic variation; Iliac artery; Intermittent claudication; Pelvis; Urinary bladder
INTRODUCTION
Understanding the anatomic variation in the internal iliac artery (IIA) is of great interest to the medical community because of the potential effects vascular compromise can have in this region. For example, IIA occlusive disease and IIA stenosis can result in thigh and buttock claudication, which is a cramping pain that usually occurs due to occlusion of an artery [1]. Lower extremity arterial disease is a disease that can be defined as either distal or proximal, where the proximal often results in hip and thigh pain [2]. Symptoms can also persist after procedures such as embolization. Buttock and thigh claudication can sometimes occur after endovascular aortic repair (EVAR), a procedure used to repair aortic aneurysms [3]. Buttock claudication can occur after treatment of a common iliac or internal iliac artery aneurysm [4]. In one study, this complication occurred in 40.7% of patients [3]. The internal iliac artery is often embolized to prevent a type II endoleak when treating a common iliac artery aneurysm [5]. Although symptoms are rare after coil embolization, understanding possible IIA variation patterns can give clinicians a glimpse into why symptoms are emerging in some patients and not others post operation. It also can be helpful in determining the reasons for gait differences among patients with peripheral artery disease (PAD) [6] found that patients with PAD had shorter step lengths and walked at a slower speed that the control group. This decrease in speed was generally not associated with claudication. Variation can account for gait differences depending on which arteries the PAD affects.
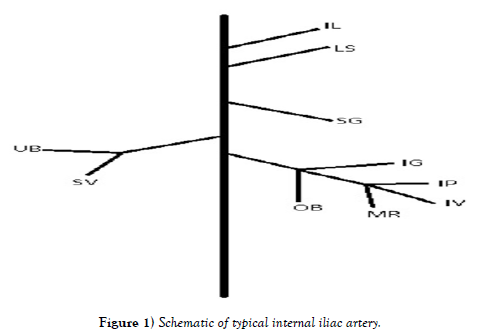
We have documented variations in the anterior division of the IIA, which contains the inferior gluteal, internal pudendal, middle rectal, obturator, umbilical, superior vesical, inferior vesical, and uterine arteries. Some variations, although minor, were also studied in the posterior division of the IIA, which includes the superior gluteal, lumbosacral, and iliolumbar arteries [7] described variation of the iliac arteries where 62.3% of the posterior division arteries arose from the common trunk [7] also described variations in lengths from the common trunk, the width of the vessels, and position of branching points for the superior gluteal, lateral sacral, and iliolumbar arteries [7]. In a study of variation in the inferior gluteal, internal pudendal, and middle rectal arteries (sample size of 111 subjects) about half the subjects displayed the internal pudendal and inferior gluteal sharing a trunk, while about a fourth of the time middle rectal and internal pudendal shared a trunk [8]. In a case report an unnamed artery was observed coming off the larger common trunk which gives off the lateral sacral artery and the superior gluteal artery [9]. This was not included in most variation studies, so we include an investigation of this here. Some instances of a muscular artery branch are noted entering the iliopsoas muscle. The most common variations of this muscular branch were off the superior gluteal artery, and off the inferior gluteal artery, where they entered either the iliacus or psoas muscle. These muscles are important for flexing the hip and are important muscles for walking. If its vasculature is compromised, this can be a reason why walking may be difficult in patients with IIA occlusive disease, or IIA aneurysms following EVAR.
MATERIALS AND METHODS
Study group: Tissue samples were collected from 25 human cadavers obtained from the New York Institute of Technology College of Osteopathic Medicine Anatomy Laboratory. Because these specimens do not meet the definition of a human subject according to the IRB, they do not require IRB exempt review. The study was HIPAA compliant and adhered to the tenets of the Declaration of Helsinki. The age of the subjects ranged from 56 to 100 years (mean, 81). 10 were male, 15 were female. The cause of death was recorded as cardiovascular or cerebrovascular in 7 subjects. Deaths were otherwise owing to neoplastic causes (N 6), respiratory (N 8), renal (N 1), sepsis (N 1), or unknown (N 2). Data on premortem risk factors were not available in all cases.
Sampling: For each individual, the branching patterns of 10 arteries from both the anterior and posterior divisions of the internal iliac artery were completely dissected using standard dissection tools and observed, making our observations of approximately 250 vessels. We studied the branching patterns of the iliolumbar, lumbosacral, superior gluteal, inferior gluteal, obturator, umbilical, inferior vesical, superior vesical, middle rectal, uterine, and internal pudendal arteries, with all identities based on destinations to organs and muscles. All data is reported as numbers of occurrences out of 25.
RESULTS
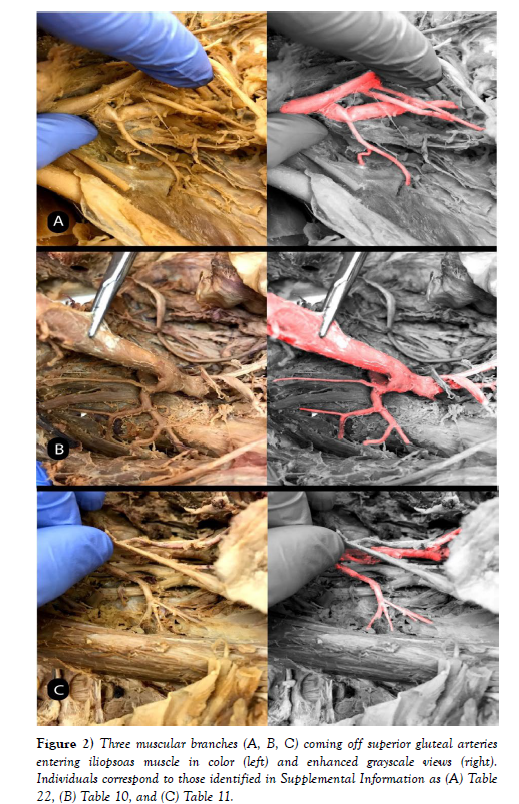
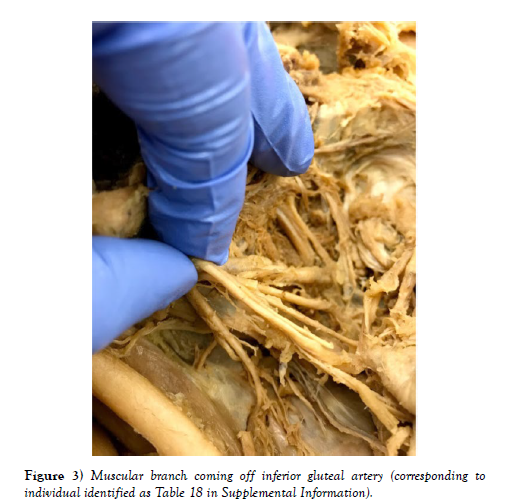
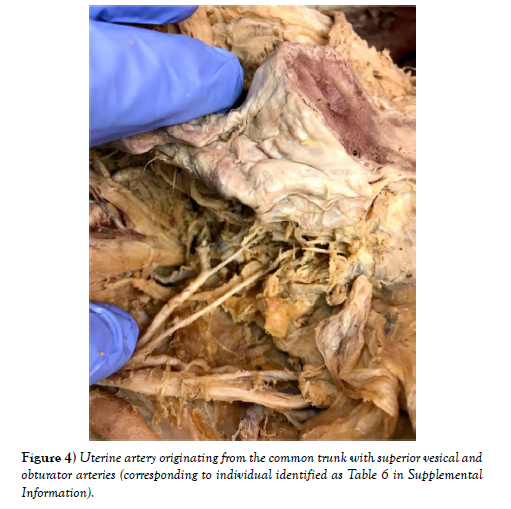
Out of 25 sections, we found muscular branches in 13 individuals (diagrams of each individual can be found in the supplemental materials). 8 were found coming off the superior gluteal artery, 3 off the inferior gluteal artery, and 2 coming off the common trunk of the umbilical and superior vesical arteries (Figures 1-4). The next most common finding was obturator artery variation, which occurred in 7 individuals. We also found significant variation in the uterine arteries, found in 6 individuals.
DISCUSSION
The muscular branch has important implications in the study of vascular disease and can lead to symptoms that are infrequently resolved. It is important for the operating surgeon to be aware of these muscular branches, because compromise of its perfusion can lead to many problems associated with muscles in the pelvic area, such as the iliopsoas. The muscular branch most frequently originated from the superior gluteal artery, which is the largest branch of the internal iliac artery. Ischemia to this artery secondary to atherosclerosis or a surgical procedure is a probable cause of gait disturbances and thigh claudication. Few studies, other than a case report [9], explore this muscular branch [9] named this branch the unnamed artery and found it on the posterior division of the IIA proximal to the lateral sacral artery. They followed the course of the vessel and found that it supplied the lateral pelvic wall. They also describe other unnamed muscular branches coming off a common trunk of the anterior division of the IIA, but the relative locations of these vessels were not reported. This was found for majority of these branches, but other variation was found as discussed earlier.
Regarding the other vessels studied, the most significant variation was that of the obturator artery, which has a variation pattern that has been thoroughly studied. We also found minor variation patterns in the internal pudendal artery in 2 cadavers, where one artery originated from a separate trunk other than the inferior gluteal, and the other from a common trunk of the umbilical and superior vesical arteries. The uterine artery had interesting branching patterns in which its origin from a bifurcation with the inferior gluteal artery, a trifurcation with the inferior and superior gluteal, and the posterior division was observed [10]. In another study [11-13] it was found that the uterine artery usually arises from the first or second branch of the internal iliac artery. We observed its origin at the bifurcation, but also found some instances of it arising from a common trunk with the superior vesical and obturator arteries. Gynecologic surgeons can use this information when operating to avoid unnecessary hemorrhaging. Vascular compromise in this artery can affect perfusion in other arteries depending on the variation pattern observed.
ACKNOWLEDGEMENT
The Associated Medical Schools of New York Anatomical Committee coordinates the whole body donations used in medical education that were sampled in this study, and we thank them for their effort in support of medical education at the New York Institute of Technology. The authors sincerely thank those who donated their bodies to science so that anatomical research could be performed. Results from such research can potentially increase mankind’s overall knowledge that can then improve patient care. Therefore, these donors and their families deserve our highest gratitude.
Conflict of Interest:
The authors declare that they have no conflict of interest.
REFERENCES
- Chung HM, Kang S-S, Shin K-M, Lee S-H, Kim SE, et al. Groin and buttock claudication associated with vascular origin due to chronic occlusion of internal iliac artery - A case report. Anesth Pain Med. 2015; 10(2):93-96.
- Mahé G, Kaladji A, Le Faucheur A, Jaquinandi V. Internal Iliac Artery Stenosis: Diagnosis and How to Manage it in 2015. Front Cardiovasc Med. 2015; (1):2-33.
- Choi HR, Park KH, Lee JH. Risk Factor Analysis for Buttock Claudication after Internal Iliac Artery Embolization with Endovascular Aortic Aneurysm Repair. Vasc Specialist Int. 2016; 32(2):44-50.
- Rayt HS, Bown MJ, Lambert KV. Buttock claudication and erectile dysfunction after internal iliac artery embolization in patients prior to endovascular aortic aneurysm repair. Cardiovasc Intervent Radiol. 2008; 31(4):728-34.
- Fontana F, Coppola A, Ferrario L. Internal Iliac Artery Embolization within EVAR Procedure: Safety, Feasibility, and Outcome. J Clin Med. 2022; 11(24):73-99.
- Szymczak M, Krupa P, Oszkinis G, Majchrzycki M. Gait pattern in patients with peripheral artery disease. BMC Geriatrics. 2018; 18:52.
- Bleich AT, Rahn DD, Wieslander CK, Wai CY, Roshanravan SM, et al. Posterior division of the internal iliac artery: Anatomic variations and clinical applications. Am J Obstet Gynecol. 2007; 197:658.e651-658.e655.
- Chase J. Variation in the Branching Pattern of the Internal Iliac Artery. In: University of North Texas Health Science Center. Fort Worth. 2016: 1-33.
- Nayak SB, Shetty P, Surendran S, Shetty SD. Duplication of Inferior Gluteal Artery and Course of Superior Gluteal Artery Through the Lumbosacral Trunk. OJHAS. 2017; 16.
- Albulescu D, Constantin C, Constantin C. Uterine artery emerging variants - angiographic aspects. Current Health Sciences Journal 2014; 40:214-216.
- Osher M, Semaan D, Osher D. The uterine arteries, anatomic variation and the implications pertaining to uterine artery embolization. J Vasc Interv Radiol 2014; 25:S143.
- Park K-M, Yang S-S, Kim Y-W, Park KB, Park HS, et al. Clinical outcomes after internal iliac artery embolization prior to endovascular aortic aneurysm repair. Surg Today 2014; 44:472-477.
- Patel SD, Perera A, Law N, Mandumula S. A novel approach to the management of a ruptured Type II endoleak following endovascular repair of an internal iliac artery aneurysm. Br J Radiol. 2011; 84(1008):e240-2.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref