Vesicovaginal Fistula. A Complication after Colposacropexy
Received: 12-Sep-2017 Accepted Date: Oct 14, 2017; Published: 16-Oct-2017
Citation: Lizarraga AC, Freixa NB, Caballero JR, et al. Vesicovaginal fistula: A complication after colposacropexy. Surg Case Rep 2017;1(1):8-10.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Mortality and morbidity due to surgical complications in the field of gynecological laparoscopy have decreased considerably during last years but early clinical diagnosis and treatment are decisive for these patients’ survival. Herein is reported a case of vesicovaginal fistula as a surgical complication of colposacropexy.
A case of a 54 years old woman who underwent a subtotal hysterectomy and promontofixation with placement of an ALYTE® Y-Mesh who developed a vesicovaginal fistula. A conservative treatment was attempted at first, but due to its failure, the patient had to be reoperated to correct the defect.
Although postoperative complications can be diminished with a good knowledge of anatomy as well as good exposure sometimes can be unavoidable and may even go unnoticed. The morbidity of patients to these unforeseen depend on a correct diagnosis ideally intraoperatively and a correct management.
Keywords
Colposacropexy; Vesicovaginal fistula; Surgical complications
Background
Fistulas are one of the most feared complications in gynecological surgery and more than 50% of them occur after hysterectomy, for benign causes. Although the true incidence is unknown, it has been estimated at around <1% [1].
The consequences of fistula can be painful and disabling, hence the importance of carrying out a proper diagnostic evaluation and repair either by conservative or surgical treatment. The main complication of surgery is the recurrence of the fistula [2].
Herein a case of a patient diagnosed with cystocele, rectocele and uterine prolapse, who underwent subtotal hysterectomy and placement of mesh for promontofixation, subsequently presenting with a posterior vesicovaginal fistula (VVF), as a post-surgical complication. With this clinical case our objective is to try to unify the steps to follow to this type of complications and to review the recent literature on the diagnosis and treatment of fistulas.
Clinical Case Presentation
A 54-year-old patient with no known drug allergies, BMI of 22 kg/m2, blood group 0+, menarche at 13 years old and carrying a Mirena IUD. Her past medical history includes previous hysteroscopic myomectomy, tubal ligation, tonsillectomy and two vaginal deliveries, one of which was a twin pregnancy.
Family history includes maternal hypertension as well as dyslipidemia.
The patient complained of having symptomatic uterine prolapse for two years. Physical examination revealed a grade 2 cystocele, a grade 2 rectocele and a grade 3 uterine prolapse with vulvar dehiscence and a normal vaginal examination. A subsequent urodynamic study was normal and the patient went on to have a laparoscopic colposacropexy.
Subtotal histerectomy and promontofixation with placement of an ALYTE® Y-Mesh were performed. The mesh was fixed by two sutures to the puborectalis, a stitch on the vaginal surface, two fastening stitches on the vaginal anterior fascia and uterosacral ligaments and a final stitch on the promontory. While using the monopolar loop for the uterine section, an accidental vaginal opening was seen, which was closed once the hysterectomy was completed by the use of three simple Polisorb 2/0 stitches.
The patient was discharged 48 hours later with antibiotic prophylaxis and a follow up appointment after a week, where the stitches would be removed and the pathology result reviewed.
Twenty days later she came for a surgical wound check-up complaining of leucorrhoea and vaginal discharge. On physical examination a 2-cm suture dehiscence covered by fibrin in the posterior fornix was visualized and which was confirmed later by digital vaginal examination where the inserted mesh was palpated. Consequently, topical treatment was recommended to reduce the inflammation and a Magnetic Resonance Imaging (MRI) was requested.
The patient came 15 days later reporting decreased leucorrhoea but had persistent pain.
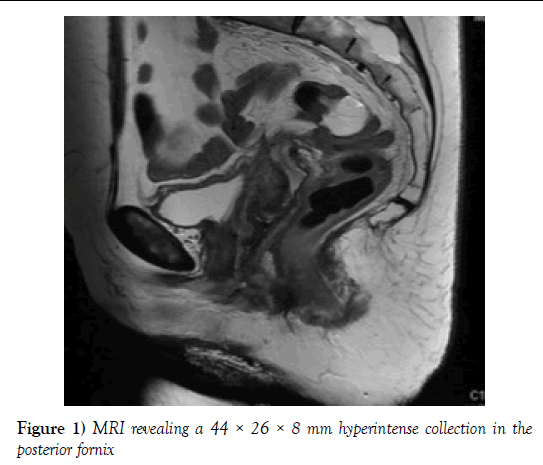
Micturition remained normal. On physical examination, decreased size of the suture dehiscence was observed. The MRI revealed a 44 × 26 × 8 mm hyperintense collection in the posterior fornix of material which had spread into the abdominal cavity (Figure 1). Due the patient’s satisfactory progress overall, it was decided to continue with the conservative treatment and a new follow-up appointment in a month was scheduled.
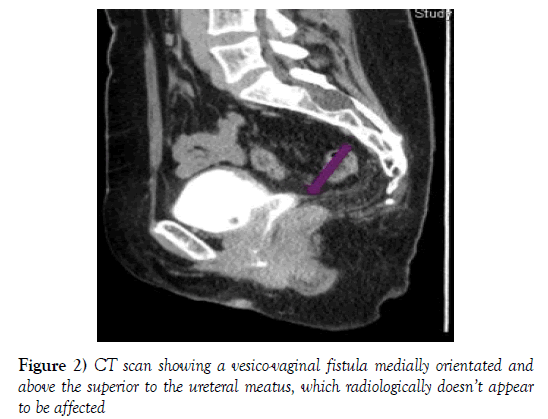
A month later the patient was systemically well and her only complaint was of stress urinary incontinence. On physical examination, the vaginal defect was minimal and inflammation had completely disappeared. Treatment with Solifenacin 10 mg and a further check-up one month later was recommended. When she came back she reported continued urinary incontinence. The examination showed that the vaginal defect had closed, with no further obvious complications from the wound site. A CT scan was requested for evaluation of the urinary tract which revealed a vesico-vaginal fistula medially orientated and above the superior to the ureteral meatus, which radiologically did not appear to be affected (Figure 2).
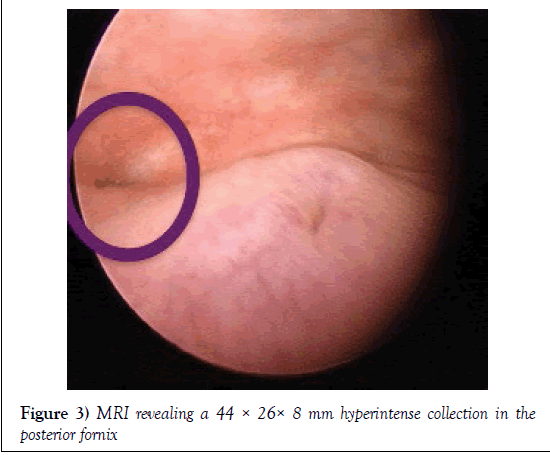
Due to these findings she underwent a cystoscopy where a small fistulous tract between fundus and posterior wall and away from both ureteral meatus was observed. Both edges and ureteral jets were normal (Figure 3) so a conservative approach was used, with the insertion of a permanent vesical catheter and further follow-up in one month with CT urography.
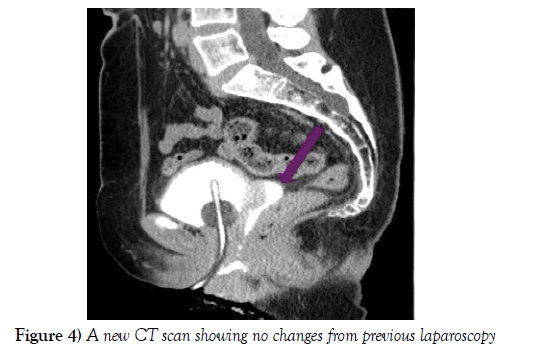
The CT did not reveal any changes (Figure 4), so a final surgical laparoscopic correction of the fistula in combination with the Urology Department was decided with prior catheterization of the left ureter. The procedure started with a cystoscopy which revealed a fistulous tract in the posterior vesical wall 2 cm away from the left ureteral meatus. A catheter was inserted into this meatus and a further one through the fistulous tract emerging in the vagina.
Laparoscopy demonstrated the absence of adhesions and inflammation, and correct placement of the mesh was observed. We proceeded to fill the bladder with saline solution mixed with methylene blue solution and a transverse incision was performed on the bladder peritoneum 1cm above the cervix to find the correct dissection plane between the bladder, and the mesh on the cervical stump. A central dissection was made towards the cervix until the mesh was seen. Once located, the dissection was continued over its surface until the posterior bladder wall was completely released. The inserted catheter was observed through the fistulous tract revealing both vaginal and bladder openings which allowed the continuation of the dissection between both orifices until healthy tissue was identified. After sectioning the catheter through the fistula, the vaginal orifice was enlarged by excising scar tissue until healthy edges were identified. This was sutured with simple absorbable stitches. The same procedure was carried out with the bladder hole, and the bladder was filled again with methylene blue solution verifying its physical integrity. Finally, an omentoplasty was performed between the two sutures to prevent recurrences. The patient was discharged four days after the surgery, once the ureteral catheter had been removed by the Urologists. One month later, a cystography was performed which was normal and the urinary catheter removed. All subsequent check-ups were normal.
The information given in this case report has the approval of our institutional IRB.
Discussion
Postoperative complications can be understood to be any deviation from the normal course [3], however a lack of consensus on a precise definition, biases around minor complications and population variations make it difficult to determine their exact incidence. One estimate of the overall complication rate in laparoscopic gynecological surgery from different series is 4/1000, with a significant risk factor being dissection near vital organs. Consequently, complications are more common in advanced surgeries such as oncological ones or those where the anatomy is distorted as in cases of deep endometriosis [4,5].
Although the incidence of urological injuries is difficult to evaluate it is estimated at <1% of patients undergoing gynecological surgeries although the number has decreased during recent years due to the use of preventive measures. Delayed diagnosis and treatment contributes to increased morbidity and mortality. The most common causes in addition to surgery; especially hysterectomies; are obstetric trauma (similar incidence), less commonly radiation and also locally advanced cancers (vaginal, cervical, endometrial). A thorough knowledge of anatomy as well as good exposure and dissection can contribute to the reduction in the rate of these injuries [6,7].
Within this broad group we must differentiate and identify bladder lesions, ureteral injuries and the presence of fistulas, whether vesicovaginal (VVF), ureterovaginal (UVF) or ureterovesicovaginal (UVVF).
Patients with VVF usually complain about continuous urinary leakage (day and night) for days or weeks after the surgical procedure which can also be aggravated during physical effort, and is often confused with stress urinary incontinence (SUI). They also may present with fever, abdominal pain, hematuria, or other constitutional symptoms secondary to hydronephrosis by ureteral obstruction or urinary extravasation and even paralytic ileus. Although they may present immediately after surgery, about 10-15% of fistulas do not present until 3-4weeks later. In some cases, patients may not present for months after with symptoms.
Risk factors such as the type of surgery, the characteristics of the patient and surgeon experience are decisive to make an early recognition of injuries and determine the success of the treatment [8,9].
Physical examination remains the most important part in making the correct diagnosis and if a VVF is suspected a test with colorants should be performed.
Imaging tests of the upper urinary tract and cystoscopy will help us to confirm the diagnosis in most cases. Cystoscopy will aid in the identification of the site of the fistula, determine the relationship with the ureteral orifices and define the size of the fistula as well as the involvement of any of the ureters.
Excretory urography, retrograde pyelography and the voiding cystourethrography provide us information of the upper tract, and it is important to note that all patients coming to the emergency room after gynecological surgery should request a delayed contrast CT to assess urinary tract.
In our case MRI was the investigation of choice, confirming the presence of the fistula in tandem with CT, as well as assessing the urinary tract [10].
The goal of treatment of these complications is not only to ensure the closure of the communication but also maintain proper bladder and vaginal capacity and adequate urinary continence.
In 10% of cases the fistula closes spontaneously after approximately half to two months with bladder catheterization and anticholinergic treatment, especially if the fistula is small, detected in time, and if no epithelialization has taken place.
Electrocoagulation, electrofulguration or sealing fibrin are options that have been considered in cases of late diagnosis with evidence of epithelialization of the fistula, although in cases of complex VVF or those with moderate inflammation such techniques have shown indeterminate results, and even the possibility of extending the defect and devitalization of adjacent tissues.
Unfortunately, in most cases this type of conservative treatment often fails, as with the case of our patient.
In cases where the diagnosis is not conducted in the first 48-72 hours it is recommended to delay the correction between three and six months allowing for the reduction of inflammation and edema, and serial checkups must be performed until the optimal date of repair is established. A hasty correction may have negative consequences.
In the case of our patient a conservative approach with antibiotics, antiinflammatory and anti-cholinergic therapy was first tried. However, the presence of a non- repair defect, likely secondary to the presence of a high degree of inflammation, required us to perform serial follow-ups until surgery was indicated, four months after the initial intervention.
There is no single optimal surgical approach for patients with VVF. Whether a vaginal, open abdominal or laparoscopic approach is used, will depend on both the clinical context of the patient and the surgeon’s experience.
The excision of the fistula should be carried out, followed by layered closure and placement of an omental flap that will maintain the bladder and vagina separated, as well as will provide neovascularization at this level.
The presence of urinary leakage immediately after the repair will be the most important negative prognostic factor [11].
The UVF usually resolve by conservative treatment with percutaneous nephrostomy or double J catheter. If this fails, or in the case of more complex fistulas, abdominal surgical repair may be required.
Finally, in UVVF, a urinary catheter should be placed until the inflammation subsides as although spontaneous healing is uncommon, conditions will be more optimal for a good correction.
Bladder injuries are another feared complication with an incidence of <3% in gynecologic surgeries, incidence being similar in laparoscopic and conventional surgery [12].
They usually occur more frequently than ureteral injuries but the intraoperative diagnosis is also higher due to gas output through the urinary catheter, the evidence of the vesical balloon or by a verification cystoscopy. Rarely they occur during entry into cavity but if this occurs, it is usually during placement of suprapubic trocar.
If detection is immediate, bladder suture with simple or continuous stitches with absorbable material must be performed and a permanent urinary catheter for 7-10 days should be placed.
Those not identified intraoperatively are usually secondary to thermal injuries during dissection, presenting as urinary ascites, pain, oliguria, nausea, vomit, abdominal distension and increased creatinine. Most cases will be resolved with a urinary catheter for 1-2 weeks and surgery should be considered in cases with no improvement.
It has been shown that over 75% of ureteral injuries are secondary to gynecological surgery with an incidence of <2%. Are about preventable injuries if anatomy and most frequently damaged locations are well known such as the infundibulum-pelvic ligament, the uterosacral and at the junction of uterine vessels. The continuous display of the ureter and ureteral dissection whenever necessary contribute to reducing these complications.
The treatment is based on placing a doble J stent or in specific cases an endto- end anastomosis or ureteral reimplantation (depending on the distance to the bladder) [13].
Conclusion
To conclude, although the real incidence and impact of all the complications reviewed during the article is not exactly known due to the imprecise definitions and variations in population the importance of conducting an early diagnosis and management is obvious. There is no single pattern or a single optimal treatment or surgical approach if needed hence the importance of individualizing each case. Resolving the defect is not only the objective but also that the patient must be guaranteed a correct capacity and quality of life.
Acknowledgement
None.
Conflict of Interest
None.
REFERENCES
- Ghosh B, Wats V, Pal DK. Comparative analysis of outcome between laparoscopic versus open surgical repair for vesico-vaginal fistula. Obstet Gynecol Sci 2016;59(6):525-29.
- Zhou L, Yang TX, Luo DY, et al. Factors influencing repair outcomes of vesicovaginal fistula: A retrospective review of 139 procedures. Urol Int 2016 Nov 24.
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications. Ann Surg 2004;240:205-13.
- Magrina JF. Complications of laparoscopic surgery. Clin Obstet Gynecol 2002;45:469-80.
- Jansen FW, Kapiteyn K, Trimbos-Kemper T, et al. Complications of laparoscopy: A prospective multicenter observational study. Br J Obstet Gynaecol 1997;104:595-600.
- Chapron C, Querleu D, Mage G, et al. Complications of gynecologic laparoscopy. Multicentric study of 7,604 laparoscopies. J Gynecol Obstet Biol Reprod 1992;21:207-13.
- Lobato JL, Andía D, Garay G, et al. Urinary tract injury in gynecologic surgery. Clin Invest Ginecol Obstet 2011;38:100-3.
- Hodges AM. Vesico-vaginal fistula associated with uterine prolapse. Br J Obstet Gynaecol 1999;106:1227-8..
- Hampel C, Neisius A, Thomas C, et al. (Vesicovaginal fistula: Incidence, etiology and phenomenology in Germany). Urologe A 2015;54(3):349-58.
- Stamatakos M, Sargedi C, Stasinou T, et al. Vesicovaginal fistula: Diagnosis and management. Indian J Surg. 2014 Apr;76(2):131-6.
- Hindocha A, Beere L, Dias S, et al. Adhesion prevention agents for gynaecological surgery: an overview of Cochrane reviews. Cochrane Database Syst Rev 2015;1:CD011254.
- Pal DK, Wats V, Ghosh B. Urologic complications following obstetrics and gynecologicai surgery: Our experience in a tertiary care hospital. Urology Annals 2016;8(1):26-30.
- Martinez JA, Castellanos V, Noyola G, et al. Diagnosis and management of vesicovaginal fistulas: twenty years of experience. Rev Mex Urol 2011; 7:200-206.