Viral nanoparticles: A drug delivery platform
Received: 25-Nov-2017 Accepted Date: Dec 25, 2017; Published: 10-Jan-2018
Citation: Alijabali AAA. Viral Nanoparticles: A drug delivery platform. J Pharm Toxicol. 2017;1(1):1-2.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Naturally-occurring nanoparticles such as viral-based particles hold great potential in the nanomedicine field. There exterior and interior can be genetically and chemically modified with various moieties to impart new functionalities. Furthermore, the internal space can be used as a reaction container or as a drug carrier for high payload depending on the shape of the viral nanoparticles. Such particles have shown no toxicity in vivo and in vitro system deeming them as safe, biocompatible and cheaper alternative to the synthetic counterparts.
Keywords
Chemotherapy; Bionanotechnology; Nanoparticles; Virus; Cancer; DNA; Chemotherapeutics; Nanotemplates
Over the past decade, there has been an increased interest in the biomaterials field for various applications. The development of cancer treatment methods (chemotherapy, radiation and hormone therapy) has saved millions of lives. However, the problem with this treatment remains the side effects (weakness, nausea, falling hair, and flu-like symptoms). Additionally, because this treatment doesn’t differentiate between healthy and cancerous cells due to the low efficacy of such drugs. This was the driving force for many scientists and researchers to engineer and design alternative drug delivery systems. One such valuable endeavor is the development of virus-based platforms for the targeted drug delivery.
Viral nanoparticles offer unmatched monodispersity platform in comparison to the synthetic nanoparticles. In addition, they are biocompatible, can be produced in large quantities and very cheap make them ideal nanoparticles. Various classes, shapes and materials composition have been reported in the literature for molecular targeting purposes. The focus of researchers in this field is in the development of plant-virus based nanoparticles to minimize the chance of the virus being infectious or causing disease to the individuals. Virus propagation is supported by the suppressive and permissive cells to cause infection. Therefore, developing plant virus drug delivery system promises to minimize the toxic side effects of the chemotherapeutics by delivering selectively to the targeted cells, reducing the immune response and interacting with mammalian cells.
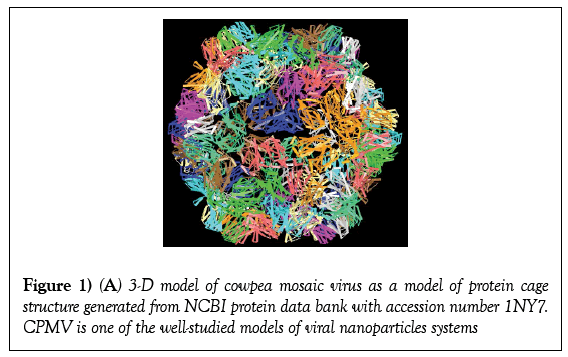
Bionanotechnology focuses on the use of derived from biological macromolecules, such as viruses, virus-like particles (VLPs) protein cages, DNA and cellular proteins make an excellent nanotemplate for functionalization with various molecules of interest [1-3]. Viruses can selfassemble both in vitro and in vivo allowing them to be used as reaction container or drug capsule [4,5]. Protein cages in general terms consist of rigid capsid that encapsidated genetic material (either DNA or RNA). This structural arrangement offers perfect spatial distribution of anchoring points [6,7]. In addition, plant viruses can be produced in large quantities with ease from infected plant leaves (Figure 1).
Figure 1: (A) 3-D model of cowpea mosaic virus as a model of protein cage structure generated from NCBI protein data bank with accession number 1NY7. CPMV is one of the well-studied models of viral nanoparticles systems
Understanding the role of the proteins in various biological functionalities and its relation to health and illness generated a wealth of structural information that allowed the manipulation of the surface of such nanoparticles. Both external and internal structures have been modified with various functionalities of interest for a broad range of applications. In particular the research effort to this end is driven into creating targeted nanoparticles “smart” with multi-functionalities to enable early detection and diagnosis of different ailments and provides at the same time high payload drugs delivered to the diseased cells precisely [8-12]. This is in contrast to the drug distribution throughout the entire body, even though the drug is needed in a specific tissue. Although major clinical success is still limited in this area viral nanoparticles offer an alternative, noninvasive therapeutic cargo carrier directed to where it is needed.
Protein cages are valuable natural nanoparticles formed from repetitive simple units to form highly organized macrostructures that are self-assembled into uniform structures. The particles are highly monodispersed (each particle looks identical) in both chemical composition (amino acids) and size [13]. The side chains of the amino acids that are present on the cages structure offer unique anchoring points for the attachment of various molecules of interest. These molecules can be tracking (dyes), targeting (antibodies, sugar molecules and peptides) that are distributed precisely on specific positions on all particles. Residue-specific strategies for the modification of the amino acids have been developed and well established. Lysine’s that bear the primary amine moieties and despite the fact that their side chains are protonated under physiological pH, they still react as nucleophiles [14]. Carbodiimide chemistry relies on the N-hydroxy Succinimide (NHS) which generates a peptide bond coupling. Furthermore, arginine resides can by modified chemically with a pyrimidine derivative [15]. The selective modification of carboxylic groups (glutamate, aspartate) or the C terminus of a protein by creating amide bonds with this terminal functionality using carbodiimide such as N-ethyl-3-N’,N’-dimethylaminopropylcarbodiimide (EDC) [16]. In addition, phenol groups on the tyrosine amino acid can react as nucleophile using diazonium salts [17]. The cysteine residues thiol side chain can be modified selectively with maleimide for the activation of thiols (cystines) [17]. All the previously described approaches rely on the use of highly reactive reagents that can selectively modify specific side chains with molecules of interest. Therefore, the generated nanoparticles resemble dendrimer with a potential of up to four different functional groups on each particle. Allowing the selective targeting to be achieved by using highly specific moieties such as RGD peptides that specifically bind to the integrin (αvβ3, αvβ5 and α5β1) that are overexpressed in almost all tumour cells. In simple terms, coating the surface of the virus particles with the targeting molecules that bonds to the cancerous cells allowing them to be target-specific. Protein cages and in particular viruses offers an elegant nanotemplates and scaffold bearing three distinct functionalities. The generated nanostructures consist of targeting, a drug of choice and tracking (imaging agents), and or immunogenic suppressor. No other synthetic form of nanoparticles offers selective multifunctionalities of endogenous amino acid side chains with functionalities of endogenous amino acid side chains with a high degree of precision on all particles.
Generally speaking, NPs biological behavior varies depending on their size, shape and composition and physical properties. Such influencing properties influence the particles circulation, tissue deposition and the toxicological effect of the NPs. Some published worked highlighted the importance of understanding the circulation, half-life stability in blood, clearance rates, organ biodistribution and immunogenicity [18]. In addition to the chemical modification of both the external and internal moieties of the particles, it is vital to assess the toxicity of such particles. As protein cages are proteinbased, they are considered as biocompatible nanostructures that don’t cause human diseases such as plant viruses or bacteriophages. Plant viruses require the cell to be suppressive and permissive in order for the infection to occur and mammalian cells do not support the cell division of plant viruses deeming them as safe nanostructures. Plant virus nanoparticles although they are infections in plants but they are most probably safe for human administration [19]. This also led to the development of VLPs as alternative route for materials synthesis which is safer than attenuated or inactivated wild-type viruses [20]. The bio-distribution and toxicity of cowpea mosaic virus (CPMV) have been assessed in vivo [21]. Qualitative biodistribution of fluorescently labeled CPMV injected intravenously into mice and in chick embryos revealed the presence of the particles in various tissues (i.e., spleen, kidney, liver, lung, stomach, small intestine, bone marrow and brain) [20,22]. In addition, bacteriophages are widely used in biopanning experiments in vivo (mice) and have been shown no overt toxicity when injected with approximately 4 × 1012 particles/kg of body weight. Furthermore, CPMV particles administered intravenously approximately 1016 particles per kilogram body weight showed no toxic effect in vivo [21]. Furthermore, the shape of the viral particles influences their distribution and clearance. It has been reported that the potato virus X accumulate in the spleen whereas smaller icosahedron viral particles such as CPMV cleared by the liver [2]. As protein cages are made of amino acids and they are protein the human immune system will attack the viral nanoparticles. Immune response, involved in CPMV injection has shown an increase in B-cells number from samples isolated from spleen [23]. Both Qβ and T7 phages injected intravenous administration induced similar B-cells count [24]. Protein cages and viral nanoparticles attack by the immune system is one of the main challenges in developing such particles for drug delivery. Covering the viral particles (i.e. CPMV) with PEG (molecular weight 2000) molecules reduced the immune response. An alternative route is to coat the surface of such particles with polymers to mask their composition and deceiving the immune system into ignoring the circulating viral nanoparticles.
Conclusion
Plant viral nanoparticles have the potential to revolutionize cancer treatment and drug targeting by creating nanoparticles that are cell-specific with very low side effects. Viral nanoparticles could potentially carry high drug payload with higher cell efficacy than traditional current available treatments.
REFERENCES
- Jaafar M, Aljabali AA, Berlanga I, et al. Structural insights into magnetic clusters grown inside virus capsids. ACS Appl Mater Interfaces 2014;6:20936-42.
- Aljabali AA, Shukla S, Lomonossoff GP, et al. CPMV-DOX delivers. Mol Pharm 2013;10:3-10.
- Sainsbury F, Saxena P, Aljabali AA, et al. Genetic engineering and characterization of Cowpea mosaic virus empty virus-like particles. Methods Mol Biol 2014;1108:139-53.
- Cuervo A, Dauden MI, Carrascosa JL. Nucleic acid packaging in viruses. Subcell Biochem 2013;68:361-94.
- Leung RLC, Robinson MDM, Ajabali AA A, et al. Monitoring the Disassembly of Virus-like Particles by 19F-NMR. J Am Chem Soc 2017;139:5277-5280.
- Koudelka KJ, Manchester M. Chemically modified viruses: principles and applications. Curr Opin Chem Biol 2010;14: 810-7.
- Portney NG, Destito G, Manchester M, et al. Hybrid assembly of CPMV viruses and surface characteristics of different mutants. Curr Top Microbiol Immunol 2009;327:59-69.
- Park Jw, Kirpotin Db, Hong K, et al. Tumor targeting using anti-her2 immunoliposomes. J Control Release 2001;74:95-113.
- Okuda T, Kawakami S, Akimoto N, et al. PEGylated lysine dendrimers for tumor-selective targeting after intravenous injection in tumor-bearing mice. J Control Release 2006;116:330-6.
- Muldoon Ll, Neuwelt Ea. BR96-DOX immunoconjugate targeting of chemotherapy in brain tumor models. J Neurooncol 2003;65:49-62.
- Kukowska-Latallo JF, Candido KA, Cao Z, et.al. Nigavekar,., JR. Nanoparticle targeting of anticancer drug improves therapeutic response in animal model of human epithelial cancer. Cancer Res 2005;65:5317-24.
- Brown WL, Mastico RA, Wu M, et al. RNA bacteriophage capsid-mediated drug delivery and epitope presentation. Intervirology 2002;45: 371-80.
- Rego JM, Yi H. Viruses as Self-Assembled Templates. Supramolecular Chemistry 2012;John Wiley & Sons, Ltd.
- Basle E, Joubert N, Pucheault M. Protein chemical modification on endogenous amino acids. Chem Biol 2010;17: 213-27.
- Oya T, Hattori N, Mizuno Y, et al. Methylglyoxal modification of protein. Chemical and immunochemical characterization of methylglyoxal-arginine adducts. J Biol Chem 1999;274:18492-502.
- Gilles Ma, Hudson AQ, Borders- Jr Cl. Stability of water-soluble carbodiimides in aqueous solution. Anal Biochem 1990;184:244-8.
- Hooker JM, Kovacs EW, Francis MB. Interior surface modification of bacteriophage MS2. J Am Chem Soc 2004;126:3718-9.
- Moghimi SM, Hunter AC, Murray JC. Nanomedicine: current status and future prospects. FASEB J 2005;19:311-30.
- Lebel ME, Chartrand K, Leclerc D. Plant Viruses as Nanoparticle-Based Vaccines and Adjuvants. Vaccines (Basel) 2015;3:620-37.
- Rae C, Koudelka, KJ, Destito G. Chemical addressability of ultraviolet-inactivated viral nanoparticles (VNPs). PLoS One 2008;3:e3315.
- Singh P, Prasuhn D, Yeh Rm. Bio-distribution, toxicity and pathology of cowpea mosaic virus nanoparticles in vivo. J Control Release 2007;120:41-50.
- Lewis JD, Destito G, Zijlstra A, et al. Viral nanoparticles as tools for intravital vascular imaging. Nat Med 2006;12:354-60.
- Gatto D, Ruedl C, Odermatt B, et al. Rapid response of marginal zone B cells to viral particles. J Immunol 2004;173:4308-16.
- Srivastava AS, Kaido T, Carrier E. Immunological factors that affect the in vivo fate of T7 phage in the mouse. J Virol Methods 2004;115:99-104.