
Page 33
Volume 3
Current Research: Integrative Medicine
Nursing Care & ICNND 2018
October 22-23, 2018
October 22-23, 2018 Madrid, Spain
46
th
World Congress on
Nursing Care, Neurology and Neuromuscular Diseases
Mike Snape, J Current Res: Int Medicine 2018, Volume 3
DOI: 10.4172/2529-797X-C2-005
The utility of concordant trend analyses in a phase 2 study in congenital and childhood onset myotonic
dystrophy type 1: A case example
Mike Snape
Amo Pharma Ltd, UK
Statement of the Problem:
Proof-of-concept clinical trials in rare diseases
such as congenital and childhood onset myotonic dystrophy type 1 are often
hampered by a lack of knowledge concerning optimal outcome measures to detect
efficacy. The concordant trends analysis offers a solution in which efficacy is
assessed by evaluating the trends on several within-study assessments, provided
the assessments are quasi- or wholly-independent. Positive findings using this
analytical technique are unlikely to arise due to chance alone.
Methodology & Theoretical Orientation:
AMO-02/tideglusib is a novel,
orally administered GSK-3β enzyme inhibitor. Overactivity of GSK3β has been
identified as a key pathophysiological feature of congenital and childhood onset
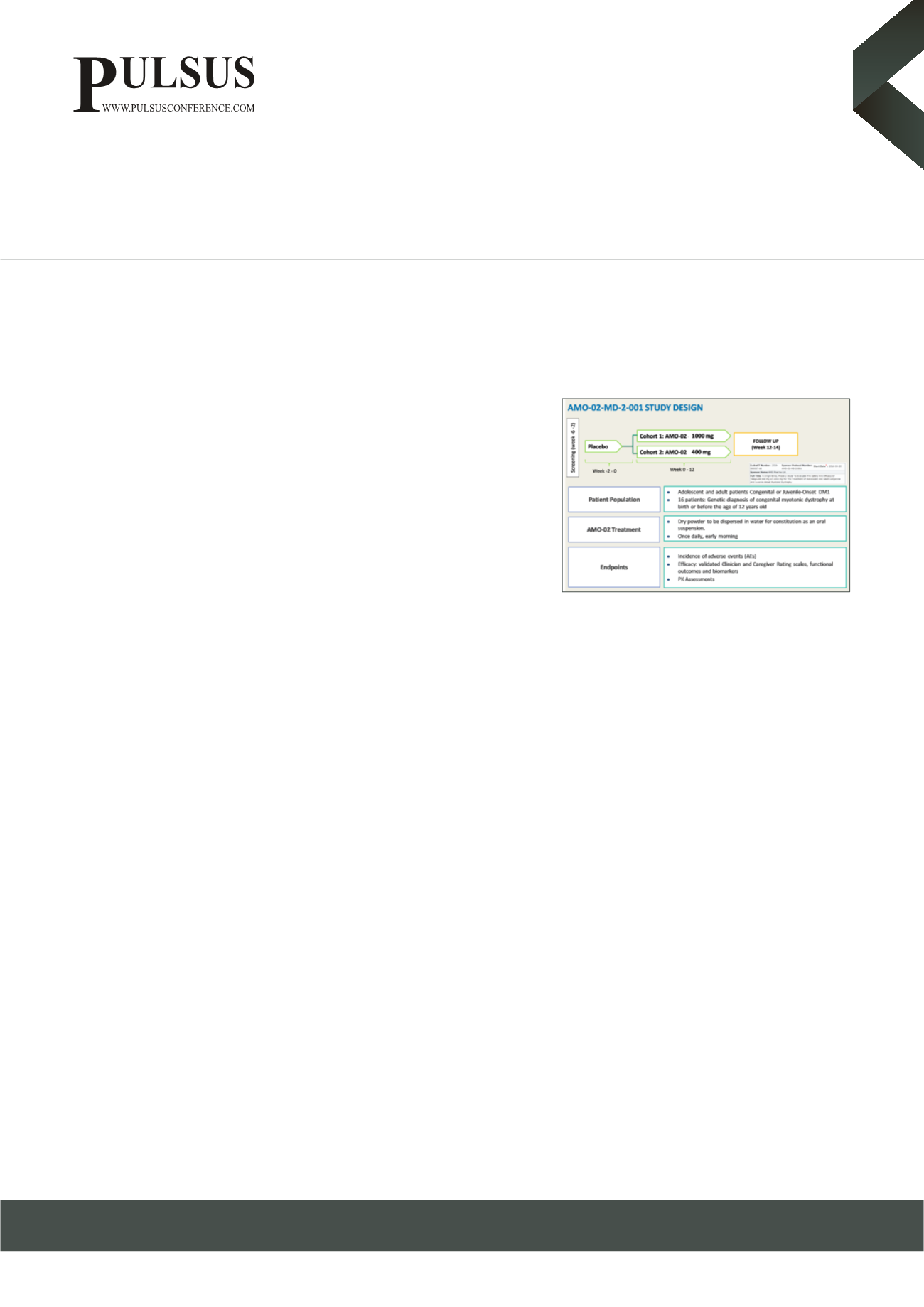
myotonic dystrophy. Accordingly, this Phase 2a clinical trial explored the utility
of AMO-02 in 16 adolescents and adult subjects with this form of myotonic dystrophy across a 12-week treatment period. Outcome
measures included disease-specific rating scales, functional/performance-based assessments, and biomarkers.
Findings:
AMO-02 rendered clinical benefit to the majority of subjects after 12 weeks of treatment. The concordant trend analysis
revealed a clear dose-response relationship that favored the 1000 mg over 400 mg dose. Four of the 10 efficacy variables (i.e. grip
strength, Clinician VAS rating scale, Caregiver Top 3 Concerns rating scale, and OSU CGI-I rating scale) differed in favor of 1000
mg over 400 mg dose and there was no worsening in the remaining six variables.
Conclusion & Significance:
The concordant trend analysis provides reiterative confidence about the study findings. This is
important since this novel therapeutic area lacks gold standard outcome measures and this study was the first clinical trial conducted
in this specific population. Accordingly, AMO-02 merits further progression in clinical development in this population of individuals
affected by early-onset myotonic dystrophy, and the 1000 mg dose may have the best prospect of establishing a consistent efficacy
signal.
Biography
Michael Snape, also known as Mike, Ph.D. is the Co-founder and Chief Executive Officer of AMO Pharma Limited. Dr. Snape is Founding Partner of Autism
Therapeutics Ltd. Dr. Snape has been involved in clinical studies in autism since 1997, and has been responsible for conceiving and executing multiple clinical
studies in autism and related neurodevelopmental disorders. Dr. Snape served as the Chief Scientific Officer of Neuropharm Group Plc from 2005 to April 9, 2010.
Dr. Snape Co-founded Neuropharm Limited. He served as Principal Scientist of Cerebrus and also served as an Associate Director of Vernalis Group plc. He has
published numerous articles in international scientific and medical journals and is named on five pharmaceutical patents.
mike.snape@amo-pharma.com















