Effect of blood transfusion on TNFα, IL-1, and IL-6 cytokine gene expression in abdominal aortic aneurysm repair patients
2 Department of Hematology, Blood transfusion research Center, High Institute for Research and Education in Transfusion Medicine, Tehran, Iran
3 Department of Biochemistry, Blood Transfusion Research Center, High Institute for Research and Education in Transfusion Medicine, Tehran, Iran
4 Department of Neurosurgery, Functional Neurosurgery Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran
5 Department of Anesthesia, Shohada Tajrish Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran, Email: faranak.behnaz@gmail.com
Received: 13-Mar-2023, Manuscript No. puljcgg-23-6224; Editor assigned: 15-Mar-2023, Pre QC No. puljcgg-23-6224 (PQ); Accepted Date: Apr 01, 2023; Reviewed: 20-Mar-2023 QC No. puljcgg-23-6224 (Q); Revised: 30-Mar-2023, Manuscript No. puljcgg-23-6224 (R),; Published: 04-Mar-2023, DOI: 10.37532.6(1).01-06
Citation: Chengini A, Valizadeh S, Samiee S. Effect of blood transfusion on TNFα, IL-1, and IL-6 cytokine gene expression in abdominal aortic aneurysm repair patients. A literature review. J clin genet genom. 2023;6(1):01-06.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
BACKGROUND: An Abdominal Aortic Aneurysm (AAA or triple A) is a localized enlargement of the aorta in the abdominal region, with a diameter of 3 cm or more and also 50% larger than normal. AAA is the most common form of aortic aneurysm, about 85% of which occur below the kidneys, at the level, or above the kidneys. In case of aortic rupture, the patient's mortality rate will be 85% to 90%. It is estimated that about 0.2% of blood recipients have a systemic inflammatory response. Considering the vital need of aortic aneurysm patients for blood transfusion, we evaluated the gene expression changes of inflammatory cytokines such as interleukin 1 and 6 (IL-1 and 6) and Tumor Necrosis Factor α (TNF-α) in these patients before anesthesia, after anesthesia, at different intervals after transfusion until the patient's discharge time.
MATERIALS AND METHODS: On the time intervals of before anesthesia, after anesthesia, and before blood transfusion in the operating room, 24 hours and 48 hours after blood transfusion (pact cells) in the ICU and surgery department, and at the time of discharge, gene expression sampling was performed. The samples were taken and the total RNA was extracted. To quantitatively measure the genes of transcription factors, Real-time PCR was performed. T-test was used to analyze the gene expression after calculating fold change.
RESULTS: IL-1 gene expression increased in 24 hours after blood transfusion (24 h) (P=0.0376) and discharge time (P=0.0301), after anesthesia (AA) (P=0.235), and 72 hours after blood transfusion (P=0.1667). IL-6 gene expression changes after blood transfusion showed increased expression 24 hours after blood transfusion(P=0.046), and at the time of discharge (P=0.0473). TNF-α gene expression increased after anesthesia (AA) (P=0.699), in 24 hours after blood transfusion (24 h) (P=0.0625), 72 hours after blood transfusion (P=0.0121), and discharge time (P=0.0141).
CONCLUSION: These changes showed IL-1, IL-6, and TNF-α gene expression increased 24 h after transfusion and upon discharge.
Key Words
Abdominal aortic aneurysm; TNFα, IL1; IL6; Gene expression
Introduction
Abdominal Aortic Aneurysm (AAA or triple A) is a localized enlargement of the aorta in the abdominal region, with a diameter of 3 cm or more and also 50% larger than normal. These patients usually do not have any symptoms, but abdominal, back, or leg pain may occur during rupture. AAA is the most common form of aortic aneurysm, about 85% of which occur below the kidneys, at the level, or above the kidneys [1]. The risk of rupture among those with a diameter of an aneurysm between 5.5 cm and 7 cm, is about 10%, while for those with an aneurysm diameter of more than 7 cm, this risk has reached about 33%, and in case of aortic rupture, the patient's mortality rate will be 85% to 90%. In 2013, the Aortic aneurysm mortality rate reached the number of 168,200 people [2]. Continuous bleeding and coagulopathy are one of the main causes of death in these patients [3]. Therefore, blood transfusion and its products in these patients are very important in their survival [4]. Although blood transfusion is a lifesaver for these patients, it is associated with complications such as immune system suppression or Transfusion-Related Immunomodulation (TRIM). One of these complications of TRIM syndrome is related to allogeneic blood transfusion. The clinical existence, mechanism, and clinical importance of TRIM in humans have been much disputed in the literature [5]. It is estimated that about 0.2% of blood recipients have a Systemic Inflammatory Response Syndrome (SIRS). Transfusion of blood products has endothelial-activated factors that may cause inflammatory reactions within the vascular compartment, potentially leading to a systemic inflammatory reaction. After the storage of all blood products, an increase in inflammatory mediators from endothelial cells has been observed [6]. General anesthesia, and surgical and trauma treatments can cause post-operative stress and post-operative immunosuppression and suppress the immune system. Even for cancer patients, it may exacerbate the existing situation [7]. Tumor Necrosis Factor alfa (TNF-α) and Interleukin-1 (IL-1) mediate the innate immune response. TNF-α has multiple biological activities, including inflammation, and anti-tumor immune functions. In addition, TNF-α has multiple anti-inflammatory functions due to synergy with other bioactive substances [8]. Interleukin 6 and Interleukin 8, as two important cytokines in activating antiinflammatory responses, can cause the secretion and chemotaxis of inflammatory cells and activate become inflammatory cells. Increasing their activities can increase the removal of bacteria, and increase antiinflammatory responses, and wound healing abilities in patients [9]. Some studies have shown that TNF-α levels are significantly increased after surgery and blood transfusion. Interleukin 10 has also increased after blood transfusion and surgery, which may prevent the activation of T lymphocytes, and even the release of some cytokines, reducing the activity of the immune system and suppressing it [9, 10]. Other studies have presented that blood transfusion did not change IL- 6 after surgery and blood transfusion compared to before surgery and blood transfusion did not cause an IL-8 decrease and IL-10 and TNF α increase, which may reduce immune system suppression after blood transfusion [10].
Considering the aortic aneurysm patients great need for blood transfusion, we determined the gene expression changes of inflammatory cytokines such as Interleukin 1 and Interleukin 6 (IL-1 and IL-6) and Tumor Necrosis Factor α (TNF-α) in these patients before anesthesia, after anesthesia, at different intervals after transfusion until the patient's discharge time [11, 12].
Aims
This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was approved by the Ethics Committee of the Higher Institute of Blood Transfusion Medicine with an ethics code (IR.TMI.REC.1397.007) and all patients individually filled out and signed the informed consent form and the amount of blood reserved according to the guideline before sampling.
Materials and Methods
Inclusion criteria
Patients diagnosed with an abdominal aortic aneurysm requiring surgical repair were admitted to Shahada Tajrish Hospital from January 2018 to August 2020. Patients with hemoglobin levels above 9 and normal coagulation profile tests were entered into this semiexperimental study. At the same time, patients with heart ejection fraction below 50% to 55% must tolerate anesthesia by following the proper principles according to the guidelines prepared in the hospital [13].
Exclusion criteria
Patients with histories of blood transfusion and its products, administration of drugs affecting the immune system, and suffering from diseases affecting the immune system (i.e., including immunodeficiency and connective tissue diseases such as Marfan, malignancy, surgery, and diabetes) were excluded. All patients had intraoperative care including invasive blood pressure measurement, Central Venous Pressure Measurement (CVP), Oxygen saturation (SpO2) with pulse oximetry, Electrocardiography (ECG), and Bi-Spectral Index (BIS). All patients were given midazolam (0.01 mg/kg), fentanyl (0.2 μg/kg), lidocaine (1.5 mg/kg), etomidate (0.2 mg/kg), and cisatracurium (0.2 mg/kg) for induction of anesthesia. Then we used sevoflurane 1% and cisatracurium to maintain anesthesia, also fentanyl was administered every 45 minutes.
After intubation, the CV line and arterial line were placed for all patients. Surgery was performed by one surgeon and one surgical team. Aortic clamp time was 40 minutes to 45 minutes in all patients. Red Blood Cell (RBC) transfusion was started in all anemic patients from the time of the aortic clamp in order not to face a drop in blood pressure during the aortic declamp. Samples were taken from each patient at different determined times. After performing real-time PCR using Ct obtained for the relevant genes. We obtained the opinion and GAPDH reference gene, the amount of Ct∆, Ct∆∆ and Ct∆∆-2 or Fold Change, which was directly proportional to the value of the gene or its copy number. We compared the fold change of patients at different times through a T-test. The mean, standard deviation, and P value were calculated, and considering P<0.05, it was determined whether or not the difference in the data was significant. Then, all patients were assessed for infection, length of hospitalization, and mortality upon discharge.
Sample collection and transfer: after obtaining written consent on 5 occasions, before anesthesia, after anesthesia, and before blood transfusion in the operating room, 24 hours and 48 hours after RBCs transfusion in the ICU and surgery department and at the time of discharge, gene expression sampling was collected. 6 ml of blood was taken from each patient in tubes containing K2-EDTA. Then the tubes were transferred to the laboratory in less than an hour, maintaining the cold chain, the samples were centrifuged at 3000 g for 10 minutes, and all their plasma was removed and frozen at (70°C) for further studies.
RNA isolation and quantitative real-time PCR
Homogenized buffy coats were extracted according to their manual with slight modifications. Briefly, after 5 minute incubation at room temperature, RNA was extracted by double chloroform and precipitate with isopropanol. RNA was washed 2 times with 75% nuclease-free ethanol and dissolved in nuclease-free water. Its quality and quantity were further evaluated by nanodrop spectrophotometry and 1% agarose gel electrophoresis. Qualified RNA was reverse transcribed by Revert Aid First-strand cDNA Synthesis kit (Thermo Fisher USA). We choose relative quantification for gene expression analysis of transcriptional factor by the Real-time PCR Sybr green method. Briefly, Quantifast Sybr Green master mix (Qiagen, Germany) was prepared at a volume of 20 µL, including 10 µL SYBR Green, l µL primer, 7 µL double distilled water, and 2 µL cDNA. The Glyceraldehyde 3-Phosphate Dehydrogenase (GAPDH) housekeeping gene was used as an internal control to normalize the data. The PCR program included an activation phase of 95°C for 5 minutes of one cycle, an amplification phase of 45 cycles at 95°C for 15 seconds, and 60°C for 35 seconds.
Data analysis
For statistical analysis, Graph Pad Prism 9.0.0 (121) software was used, and the Kolmogorov-Smirnov test was used to show the normal distribution of data. T-test was used to analyze gene expression after calculating fold change through Ct∆∆-2. P value<0.05 was considered as the significance of the findings.
Results
Out of 20 aortic aneurysm patients in this research, 16 (80%) were men and 4 (20%) were women. The age range of men was 43 to 86 with an average of 68 ± 11.6 and the age range of women was 45 to 81 with an average of 68 ± 16.3. The age range of all participants was 43 to 86 with an average of 68 ± 12.6.
After surgery, patients were transferred to the ICU and after that to the surgery ward. One case of mortality was observed after surgery due to MI. No infection was observed in any of the patients who survived. The duration of hospitalization in the ICU department was from 1 day to 4 days with an average of 2.33 ± 1 and in the surgery ward was 2 days to 5 days with an average of 3.12 ± 1. The total duration of hospitalization was 4 days to 7 days with an average of 5.4 ± 1.
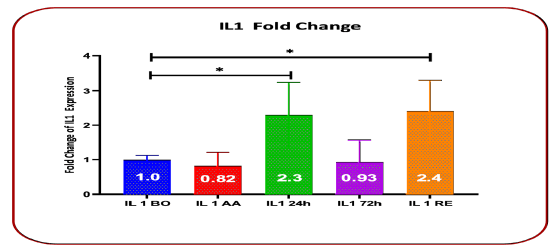
IL-1 gene expression increased in 24 hours after blood transfusion (24 h)with a fold change of 2.3 (P=0.0376) and discharge time (RE) with a fold change of 2.4 (P=0.0301). But its expression changes after Anesthesia (AA) were observed with a fold change of 0.823 (P=0.235), and 72 hours after blood transfusion (72 h) with a fold change of 0.93 (P=0.1667) (Table 1 and Figure 1).
TABLE 1 Comparison of Fold Chang changes in IL-1 gene expression before surgery, after anesthesia, 24 hours and 72 hours after transfusion, and upon discharge
| Gene expression IL | Fold Change | Standard deviation | P value |
|---|---|---|---|
| Before surgery | 1 | 0.124 | |
| After anesthesia | 0.823 | 0.39 | 0.235 |
| 24h after blood transfusion | 0.3 | 0.93 | 0.0376* |
| 72h after blood transfusion | 0.93 | 0.64 | 0.1667 |
| Discharge time | 2.4 | 0.89 | 0.0301* |
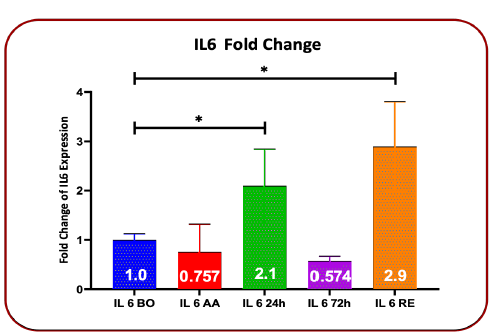
The results of the fold changes in IL-6 gene expression after blood transfusion compared to before blood transfusion showed that after anesthesia the fold change was 0.757 (P=0.4350), 24 hours after blood transfusion the fold change was 2.1 (P=0.046), 72 hours after blood transfusion, the fold change was 0.574 (P=0.166) and at the time of discharge, the fold change was 2.72 (P=0.0473). These changes showed a decrease after anesthesia and 72 hours after blood transfusion (P>0.05), and increased expression was seen 24 hours after blood transfusion and at the time of discharge (P<0.05) (Table-2 and Figure 2).
TABLE 2 Comparison of Fold Chang changes in IL-6 gene expression before surgery, after anesthesia, 24 hours and 72 hours after transfusion, and upon discharge time
| Gene expression IL6 | Fold Change | Standard deviation | P value |
|---|---|---|---|
| Before surgery | 1 | 0.121 | |
| After anesthesia | 0.757 | 0.56 | 0.435 |
| 24h after blood transfusion | 2.1 | 0.74 | 0.0476* |
| 72h after blood transfusion | 0.574 | 0.092 | 0.1667 |
| Discharge time | 2.72 | 0.91 | 0.0473* |
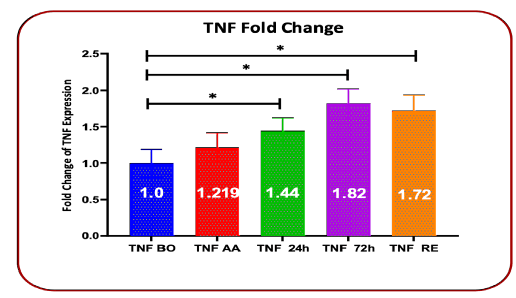
Gene expression fold change showed an increase in TNF-α gene expression in all stages of evaluation, which increased expression in 24 hours, 72 hours after transfusion, and at the time of discharge, with P=0.0256, P=0.0121, and P=0.0141 respectively were statistically significant, while it was not significant in the time after anesthesia with p=0.0699 (Table-3 and Figure 3).
TABLE 3 Comparison of Fold Chang changes in TNF-α gene expression before surgery, after anesthesia, 24 hours and 72 hours after transfusion, and upon discharge time
Gene expression TNF |
Fold Change | Standard deviation | P value |
|---|---|---|---|
Before surgery |
1 | 0.188 | |
After anesthesia |
0.198 | 1.219 | 0.0699 |
| 24h after blood transfusion * | 1.444 | 0.178 | 0.0256* |
| 72h after blood transfusion | 1.821 | 0.2 | 0.0121* |
Discharge time |
1.72 | 0.125 | 0.0141* time |
This study showed no correlation between the age and gender of the patient receiving RBCs in the expression level of the cytokines TNFα, IL6, and IL1. Also, there was no significant correlation between the expression level of the mentioned genes and age using the Spearman method (P>0.05).
Discussion
The results of the present study in the expression of TNF-α, IL-1, and IL-6 genes after RBC transfusion showed an increase in the expression of all three genes. This rise in IL-1, in 24 hours after receiving blood (P=0.0376) and at the time of discharge (P=0.0301) was significant. IL-6 was similar to IL-1, but in the case of TNF-α, this rise was observed at all four times after anesthesia, 24 hours and 72 hours after transfusion, and at the time of patient discharge, which was not statistically significant after anesthesia, but at 24 hours and 72 hours after receiving blood and discharge time was statistically significant (P<0.05).
A study conducted by Torrance et al. presented an increase in TNF-α gene expression (P=0.015) in patients with severe trauma 24 hours after receiving blood [11]. The study of TNF-α level in patients plasma using the ELISA method by Jian-Rong and Colleagues was also consistent with our findings, but in patients with gastrointestinal surgery, it was associated with a decrease in 24 hours after receiving blood. The study conducted by Carol Dani and colleagues in premature infants showed an increase in IL-1 in serum and plasma and no change in TNF-α and IL-6 after blood transfusion [12]. The possible mechanism for the increase of inflammatory cytokines can be considered due to the inflammatory factors present in the blood unit that arise as a result of storage and related to the presence of WBCs, HLA, Heme, Iron vesicles, which even prevent the increase of inflammatory cytokines, the use of fresh blood and leukocyte reduction has been suggested [7,13].
Cytokines are products that are secreted outside the cell and affect the function of other cells and organs of the body. The main cytokines of the immune system are lymphocytes and monokines, which are secreted by lymphocytes and monocytes. According to the way of production, characteristics, quality of effect, and type of target cells, some of these substances are called interleukin, some interferon, tumor necrosis factor, and some chemokine. These substances play an essential role in the production of specific and non-specific responses, and the development and evolution of humoral and cellular responses. Cytokines are a diverse group of proteins, produced during non-specific and specific immune action and play a role in the formation and regulation of immune and inflammatory responses. In non-specific immunity, substances such as Lipopolysaccharide (LPS) or viral products with double-stranded RNA directly induce mononuclear phagocytes to secrete cytokines. On the other hand, T-cell cytokines are produced following the specific recognition of foreign antigens. However, this distinction is not definitive, because cytokines produced by one type of cell often control the production of cytokines by other cells, and cytokine production is a temporary self-limiting phenomenon. In general, cytokines do not exist in the form of pre-made molecules and their production is done by starting gene transcription, which is usually temporary and mRNA coding for cytokines is unstable. They are usually secreted quickly after being produced. Many cytokines are produced by several different types of cells. For this reason, researchers prefer the word cytokine to monokine and lymphokine. Cytokines act on different cells and this property is called Pleotropism. The idea that cytokines are produced by leukocytes and act on leukocytes (interleukins) is not acceptable. Cytokines often exert several different effects on the same cell. Some of these effects may happen simultaneously and some may happen over time (minutes, hours, or days) [14]. A cytokine usually affects the production of other cytokines, leading to the start of a cascade where the second and third cytokines can manifest the biological effects of the first cytokine. Cytokines often have a reinforcing or opposing effect on each other [15]. In 2010, Leal-Noval et al. investigated the predictive effect of T-CD4+ lymphocyte cytokines on blood transfusion in cardiac surgery and studied the production of TNF-α, IL10, and TH by flow cytometry. They concluded that a low preoperative Th1 immune response, as assessed by interleukin-10 and TNF-α producing CD4+ T-helper, was associated with a higher transfusion rate [16]. It is not clear how low preoperative levels of TNF-α related to preoperative bleeding, but there is some clinical evidence of an association between the inflammatory response and bleeding. Changes in TNF-α genetic polymorphisms, which lead to low levels of TNF-α have been associated with bleeding in other areas such as cardiac surgery and aneurysmal subarachnoid hemorrhage [17].
Aba-cilar et al. investigated TNF-α level after the operation and observed its increase to more than 20 pg/mL along with the increase in the amount of bleeding and prolongation of the intubation time [18]. We also observed increased gene expression of TNF-α during 48 hours after transfusion until patients’ discharge. IL-1 and IL-6 increased 24 hours after transfusion and at the time of discharge. The difference was significant compared to the time before surgery. Considering that infection was not seen in our patients, we postulate that the increase of these cytokines is associated with transfusion in patients.
In 2002, A. D. Santini et al. investigated the effect of blood transfusion during radiotherapy on the immune system function of cervical cancer patients, emphasizing the role of IL-10. They divided 15 patients into two groups with blood transfusion and without blood transfusion and the amount of T-CD4+, T-CD8+, and Nk B-Cell populations were examined before, during, and after blood transfusion. A significant increase in the number of T-CD8+, T-CD3+, and Nk was observed after blood transfusion, and IL10 was observed only in the plasma of patients with blood transfusion [19].
This study showed no correlation between the age and gender of the patient receiving RBCs in the expression level of the cytokines TNFα, IL6, and IL1. Also, there was no significant correlation between the expression level of the mentioned genes and age (P>0.05).
But some studies have shown that both types of intravascular aortic aneurysm treatment and endovascular and conventional repair of an abdominal aortic aneurysm with significant amounts of primary acute systemic inflammatory responses and acute phase reactions (CRP synthesis, fever, and leukocytosis), shedding of Intracellular Adhesion Molecule 1 (ICAM-1) solution, IL-6 (without TNF-α or IL-8) and the release of complement products are associated [20]. Even some other studies have shown that high levels of TNF-α are associated with poor outcomes in the open repair of abdominal aortic aneurysms [21, 22]. The level of IL-6 plasma levels increased after placing the endoluminal graft in the abdominal aorta in all patients on the first day after the operation. Post Implantation Syndrome (PIS) is a relatively common complication of Abdominal Endovascular Aneurysm Repair (EVAR) used to treat AAAs and is associated with features of a systemic inflammatory response and prolonged hospitalization time.
But Arnaoutoglou's colleagues examined IL-1, IL-6, and TNF-α in aortic aneurysm repair surgery patients with and without PIS and did not observe any difference between the two groups. Although they also examined complete blood count, fibrinogen, high sensitivity Creactive protein [22].
In this way, the increase in inflammatory cytokines may not be considered directly due to blood transfusion because the mechanism of aortic aneurysm formation and even repair of aortic vessels is accompanied by the increase of inflammatory cytokines such as interleukin 6 and TNF-α.
Conclusion
These changes showed IL-1, IL-6, and TNF-α gene expression increased 24 h after transfusion and upon discharge.
Limitations
Tissue damage, trauma, and even the type of graft created can cause the release of inflammatory factors. On the other hand, with a low number of patients due to limited financial resources.
Ethical Approval
This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was approved by the Ethics Committee of the Higher Institute of Blood Transfusion Medicine with an ethics code (IR.TMI.REC.1397.007) and all patients individually filled out and signed the informed consent form and the amount of blood reserved according to the guideline before sampling.
Acknowledgment
Authors have no conflict of interest to declare. The authors certify that they and their family members have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript. The authors have no conflict of interest to declare.
Declaration
•Chegini designed the study concept and performed theoretical research, revised the lab study, wrote the final manuscript.
•S. Valizadeh did the lab work, collected the data, documented the information.
•S. Samiee did the lab work, collected the data, documented the information.
•S. Zandpazandi performed the statistical analysis and critically revised the final manuscript.
•F. Behnaz, corresponding author, did patient selection and preparation, supervised the statistical analysis, wrote the first draft of the manuscript.
Data Availability
The data collected for this study was from the patients who were willing to participate in this research study and gave informed written consent. Since there is no data registry for this information in Iran, all data is collected and documented by the authors mentioned, personally. We can send Inflammation, data and we can authorize private access to this data via a private link.
References
- Sakalihasan N, Limet R, Defawe OD. Abdominal aortic aneurysm. The Lancet. 2005;365(9470):1577-89.
- Thomas DM, Hulten EA, Ellis ST, et al. Open versus endovascular repair of abdominal aortic aneurysm in the elective and emergent setting in a pooled population of 37,781 patients: a systematic review and meta-analysis. Int Sch Res Not. 2014.
- Hardy JF, De Moerloose P, Samama CM. The coagulopathy of massive transfusion. Vox Sang. 200;89(3):123-7.
- Johansson PI, Stensballe J, Rosenberg I, et al. Proactive administration of platelets and plasma for patients with a ruptured abdominal aortic aneurysm: evaluating a change in transfusion practice. Transfusion. 2007;47(4):593-8.
- DA F, David Sturgess MB, Afracma G, et al. Transfusion implications in a COVID-19 era. Australasian Anaesthesia. 2021;1:148.
- Urner M, Herrmann IK, Buddeberg F, et al. Effects of blood products on inflammatory response in endothelial cells in vitro. PloS one. 2012;7(3):e33403.
- Guo JR, Xu F, Jin XJ, et al. Impact of allogenic and autologous transfusion on immune function in patients with tumors. Asian Pac. J. Cancer Prev. 2014;15(1):467-74.
- Chen X, Oppenheim JJ. TNF-α: an activator of CD4+ FoxP3+ TNFR2+ regulatory T cells. TNF Pathophysiol. 2010;11:119-34.
- Schroeder S, von Spiegel T, Stuber F, et al. Interleukin-6 enhancement after direct autologous retransfusion of shed thoracic blood does not influence haemodynamic stability following coronary artery bypass grafting. Thorac. Cardiovasc. Surg. 2007;55(2):68-72.
- Xing YL, Wang YC. Influence of autologous and homologous blood transfusion on interleukins and tumor necrosis factor-α in peri-operative patients with esophageal cancer. Asian Pac. J. Cancer Prev. 2014;15(18):7831-4.
- Torrance HD, Brohi K, Pearse RM, et al. Association between gene expression biomarkers of immunosuppression and blood transfusion in severely injured polytrauma patients. Ann. Surg. 2015;261(4):751-9.
- Dani C, Poggi C, Gozzini E, et al. Red blood cell transfusions can induce proinflammatory cytokines in preterm infants. Transfusion. 2017;57(5):1304-10.
- Oppenheim JJ. Cytokines: past, present, and future. Int j hematol. 2001;74:3-8.
- Jaffer U, Wade RG, Gourlay T. Cytokines in the systemic inflammatory response syndrome: a reviews. HSR proc intensive care cardiovasc anesth. 2010;2(3):161.
- Leal-Noval SR, Muñoz-Gómez M, Arellano V, et al. Influence of red blood cell transfusion on CD4+ T-helper cells immune response in patients undergoing cardiac surgery. J Surg Res. 2010;164(1):43-9.
- Chegini A, Valizadeh S, Samiee S, et al. Effect of blood transfusion on TNFα, IL-1, and IL-6 cytokine gene expression in abdominal aortic aneurysm repair patients.
- Abacilar F, Dogan OF, Duman U, et al. The changes and effects of the plasma levels of tumor necrosis factor after coronary artery bypass surgery with cardiopulmonary bypass. Heart Surg Forum. 2006;9(4)E703-9.
- Santin AD, Bellone S, Palmieri M, et al. Effect of blood transfusion during radiotherapy on the immune function of patients with cancer of the uterine cervix: role of interleukin-10. Int J Radiat Oncol Biol Phys. 2002;54(5):1345-55.
- Galle C, De Maertelaer V, Motte S, et al. Early inflammatory response after elective abdominal aortic aneurysm repair: a comparison between endovascular procedure and conventional surgery. J vasc surg. 2000;32(2):234-46.
- Pärsson HN, Nässberger L, Norgren L. Inflammatory response to aorto-bifemoral graft surgery. International Angiology: a Journal of the International Union of Angiology. 1997;16(1):55-64.
- Roumen RM, Hendriks T, van der Ven-Jongekrijg J, et al. Cytokine patterns in patients after major vascular surgery, hemorrhagic shock, and severe blunt trauma. Relation with subsequent adult respiratory distress syndrome and multiple organ failure. Ann Surg. 1993;218(6):769.
- Asghar U, Qadir A, Hanif A, et al. Safety and Efficiency of Endovascular Treatment of Open Versus Endovascular Repair of Abdominal Aortic Aneurysm. Pak. J of med Health Sci. 2016;10(4):1143-6.