Multiple central giant cell granuloma’s in the mandible - a rare presentation with literature review.
Received: 03-Jul-2022, Manuscript No. puldcr-22-5228; Editor assigned: 05-Jul-2022, Pre QC No. puldcr-22-5228 (PQ); Accepted Date: Jul 25, 2022; Reviewed: 19-Jul-2022 QC No. puldcr-22-5228 (Q); Revised: 22-Jul-2022, Manuscript No. puldcr-22-5228 (R); Published: 26-Jul-2022, DOI: 10.37532. puldcr-22.6.4.1-3
Citation: Abiola O, Vempaty P, Abiola H, et al. Multiple central giant cell granulomaâ??s in the mandible - A rare presentation with literature review . Dent Case Rep. 2022;6(4):01-03.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Central giant cell granulomas (CGCG) are rare, benign destructive osteolytic lesion of osteoclastic origin with variable aggressiveness that occur in the maxilla and mandible. It has a peak prevalence between the ages of 10 to 25 years old with a clear preponderance for the mandible and female population. CGCG typically presents as a solitary lesion, appearing as a multilocular radiolucency with scalloped margins and a honeycomb or soap bubble like appearance. These lesions are histologically characterised by mononuclear and multinuclear giant cells on a mesenchymal stromal background. First reported by Jaffe the lesion was coined giant cell reparative granuloma; a term no longer used as understanding of the pathogenesis develops. Genetic sequencing has shown familial association and a connection with “RAS/MAPK syndromes” as they are linked by overlapping facial features and are caused by mutations at different points along the RAS/MAPK pathway. Thirty percent of CGCG present as aggressive lesions characterised by rapid growth and bone destruction. The most common treatment is surgical curettage. However, increasing knowledge of the underlying pathogenesis has led to development of non-surgical treatments such as intralesional corticosteroid injections, therapy with calcitonin, interferon and monoclonal antibodies. This paper presents the rare case of an asymptomatic 14-year-old boy with bilateral CGCG which were noted as an incidental finding on a panoramic radiographic as part of an orthodontic assessment. It was treated successfully through surgical curettage.
Key Words
Central Giant Cell Granuloma; Malocclusion; Surgery; Peripheral ostectomy; General anaesthesia.
Introduction
Central giant cell granulomas (CGCG) are rare, benign lesions of the maxilla and mandible with varied presentations since their first report by Jaffe in 1953 [1]. Reports have shown a variable aggressive nature of these destructive osteolytic lesions which mainly afflict young adults and children between the ages of 10 years to 25 years old with a preponderance for the mandible and female population (62%) [2-4].
Usually presenting as a solitary lesion CGCG radiologically appear as a multilocular radiolucency with scalloped margins and a honeycomb or soap bubble like appearance. It has a prevalence of 0.00011% and accounts for <7% of all benign tumors of the jaws [3]. The WHO define this entity as a “localised benign but sometimes aggressive osteolytic proliferation consisting of fibrous tissue with hemorrhage and hemosiderin deposits and presence of osteoclast-like giant cells with reactive bone formation” [1].
Indicative of its neoplastic nature CGCG often develop spontaneously, although reports have shown trauma as an important etiological factor [4]. Since its first report, pathogenesis theories have included that CGCG may be an inflammatory or reactive lesion, a true tumor, or an endocrine lesion [3]. Recent developments in molecular genetics have highlighted several genetic aberrations that may help differentiate from giant cell tumor of the bone or other giant cell rich lesions [5].
Treatment options vary depending on the clinical characteristics of the lesion however surgical curettage is the most common treatment [6]. More invasive resections with peripheral ostectomy have seen lower recurrence rates although aesthetics and function are impacted further [7]. Non-surgical options have shown to reduce the size of lesions, although in many cases surgical intervention was also required [8].
Case Presentation
A 14-year-old boy presented to the orthodontist with malocclusion of teeth following a referral from his dentist. His medical status was unremarkable with no known systemic disorder. No history of trauma was elicited nor any systemic or local infections. The prenatal history was unremarkable and delivery was at full term and normal.
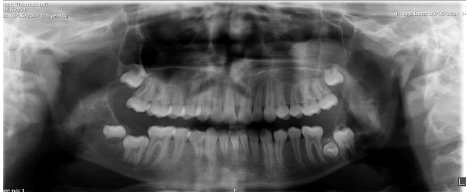
An orthopantomogram and cephalogram were taken as part of the primary investigations Figure 1. The radiograph shows two lesions on either side of the thmandible. The one on left is a well-defined, thinly corticated, multilocular radiolucency on the left anterior ramus within anatomical boundaries. Superiorly the lesion extends to the coronoid process. Posteriorly it is in contact with the mandibular canal and foramen and its inferior extent is the angle of the mandible. The right side shows a smaller well defined, thinly corticated, multilocular radiolucency immediately distal to unerupted LR8. Clinically the patient had no pain, tooth mobility, or swelling. There was no history of similar disease in any of the siblings or the parents of the affected child.
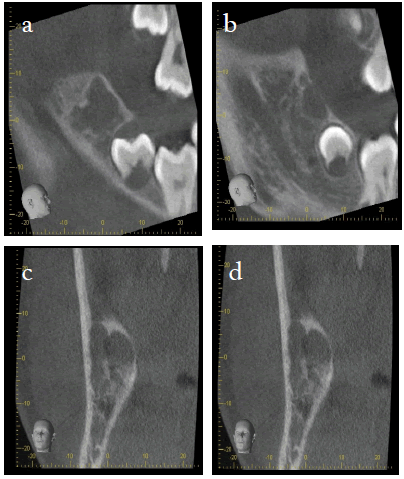
A subsequent CBCT was done to assess the three-dimensional extent of these lesions. The imaging reveals a 30 mm x 15 mm x 17 mm well-defined, corticated radiolucency of the left anterior ramus Figure 2 (2a, 2b), extending to the coronoid process. It is a single cavity with a multilocular appearance. Anteriorly it is in contact with the follicle of the unerupted most distal molar. Poster-inferiorly it is in contact with the superior wall of the inferior dental canal, with no obvious displacement or loss of its bony wall, and about 2 mm-4 mm distance from the mandibular foramen. The lesion extends along the inferior dental canal. There is cortical thinning and mild expansion of ramus laterally, with likely perforations. Lingually the cortex is thinned but intact. The anterior bony margin of the lesion is lost. The internal attenuation is consistent with soft tissue +/- fluid, but with no internal calcification.
The lesion on the right side Figure 3 (3a-3d) is a well-defined, partially corticated irregularly shaped radiolucency distal to the third molar. It is multilocular in appearance. Anteriorly it is in contact with the follicle of the unerupted most distal molar, with maintenance of the bony wall between them. It comes close but does not touch the superior wall of the ID canal. There is medial cortical thinning and slight expansion, with possible perforation. There is some thinning of the lateral cortical plate. Anteriorly there is a small perforation medial to the anterior oblique ridge. Maximum dimensions are 15 mm Supero-inferior, 13 mm medio-lateral and approximately 14 mm antero-posteriorly.
Other findings of note show the lower left third and second molar is transposed. The fact that both lesions perforated the cortex, the anterior border on the left side is destroyed and the irregular decortication of mandible on the right are suggestive of its aggressive nature. We classified it to be an aggressive lesion of mandible.
The patient underwent complete enucleation through surgical curettage of both lesions through an intra-oral approach under general anaesthesia. The lower right third molar and transposed lower left third molar were extracted after orthodontic consultation. The wound was then closed primarily. The post-operative course was uneventful. Histology of both specimens showed the characteristic mononuclear and multinuclear giant cells with spindleshaped mesenchymal stromal cells of CGCG. Therefore, a diagnosis of aggressive bilateral central giant cell granuloma was made. Preoperative blood investigations were conducted to rule out hyperparathyroidism. The blood results suggested normal calcium metabolism. Re-enquiry of family history did not reveal any syndromic predisposition. We plan to study the tissue to look for activating mutations in the RAS/MAPK pathway.
Discussion
First reported by Jaffe the lesion was coined giant cell reparative granuloma; a term no longer used as understanding of the pathogenesis develops [1,9]. The biological behavior of CGCG of the jaws ranges from asymptomatic lesions with slow growth and low recurrence rate to an aggressive pathological process and therefore is best classified as a benign neoplasm. The aggressive lesion is characterised by pain, rapid growth, root resorption, cortical perforation and a high recurrence rate. The Hillerup and Hjørting-Hansen Theory (1978) was widely accepted, and they suggested that CGCG, giant cell tumor and traumatic bone lesions are different manifestations of the same process [10]. The theory states that primary bone disease, malformation or minute trauma can lead to intraosseous hematoma; each manifestation is produced by altered blood supply [10].
Trauma has been considered as an important aetiological factor in the initiation of this lesion in predisposed subjects [11]. More recent genetic advances sees Edwards differentiate the lesions into extragnathic and gnathic variants which could be associated with syndromes like neiorfibromatosis, cardiofaciocutaneous syndromes, Noonans syndrome and LEOPARD syndrome [12,13]. These are categorised as “RAS/MAPK syndromes” as they are linked by overlapping facial features and are caused by mutations at different points along the RAS/mitogen-activated protein kinase (RAS/ MAPK) pathway which regulates cell growth, differentiation, senescence, and death [13]. Multiple CGCG is a rare but typical complication of a dysregulated RAS/MAPK pathway and shares phenotypic characteristics of cherubism. The underlying genetic mutations and sequelae distinguish the two. Mutations in SH3BP2 are associated with cherubism, whereas mutations in PTPN11 and SOS1 have been reported in people with multiple CGCG [8]. Giant cell lesions in cherubism tend to spontaneously resolve, whereas those observed in multiple CGCG can have aggressive signs and symptoms.
Aggressive lesions represent up to 30% of all CGCG and are characterised by rapid growth and bone destruction [3]. These lesions display no significant histological differences from non-aggressive lesions however they have higher recurrence rates. Clinically aggressive lesions are usually greater than 50 mm in size and can cause sensory disturbance, bone expansion, cortical perforation, tooth displacement or transposition, and root resorption [14].
Surgical curettage is the most common treatment and gives satisfactory results with recurrence rates of 11%-49%. However, treatment plans depend on location, and clinical and radiographic features [11]. Symptomatic aggressive lesions show a higher rate of recurrence [15]. Studies show more aggressive resections with peripheral ostectomy can have a recurrence rate as low as 6% although aesthetics and function are consequentially compromised [5]. Regular radiological follow-up is necessary to reveal recurrence [16]. Non-surgical management is often used for young children to avoid facial deformity and in cases where surgery is contraindicated, if the lesion has large extensions or a high recurrence rate of the aggressive variant is seen [17].
Intralesional corticosteroid injections with triamcinolone acetonide have been used since the 1980s. Literature has shown it can decrease the size of the lesion in 57% of cases although complete resolution was reported in only 10% of patients. Further surgical intervention was required in 50% of cases and 7% showed no response [8]. Corticosteroids inhibit the extracellular production of lysosomal proteases; induce apoptosis in osteoclast-like cells; inhibit transcription factors for intracellular proliferation; and induce antiangiogenic effects on endothelial cells [18]. These factors lead to inhibition of resorption, thus preventing the growth of CGCG. The discomfort caused by the injections and patient compliance are the main disadvantages with potential systemic effects especially concerning in immunocompromised and diabetic patients.
Calcitonin therapy is a viable treatment option and can completely resolve lesions although treatment usually spans 19 months to 21 months therefore is usually reserved for multiple, recurrent, or particularly aggressive lesions [19]. The binding of calcitonin to the receptor causes changes in cell structure, leading to inhibition of DNA synthesis by cells [19].
Monoclonal antibodies prevent the osteolytic process and have shown promising results in the treatment of CGCG which was trialed on the assumption giant cells present in the CGCG are analogous to osteoclasts [5]. The evidence of its use is scarce. Further studies are needed to determine its real effectiveness in treating CGCG especially when its possible side effects such as hypophosphatemia, pain in the extremities, anemia, and jaw osteonecrosis are considered [18]. CGCG can respond positively to antiangiogenic therapy after a period of adjuvant interferon alpha [20 ]. The side effects range from flu-like symptoms to complaints such as hypothyroidism and depression [8]. Some cases required dose adjustment or cessation of administration. In addition to toxicity, treatment is long. The non-surgical treatment modalities discussed can be effective as an alternative in the management of CGCG, although 40% of patients required further surgical treatment [8].
Conclusion
Early detection and treatment aid in successful outcomes for patients. The nature of aggressive lesions prompts justification for surgical management. Significant progress is being made to understand the pathogenesis of CGCG. These developments have aided the progression of non-surgical management of CGCG which has provided positive results, especially with regard to reducing the size of the lesion. Further studies are needed if these are to be first-line therapies in all cases due to the side effects and current need for surgical supplementation. We feel that delineation of aggressive and non-aggressive lesions with more comprehensive scientific evidence is much needed and can help in making a sound treatment plan and looking after the young population who are affected by this condition.
References
- Orhan E, Erol S, Deren O, et al. Idiopathic bilateral central giant cell reparative granuloma of jaws: a case report and literature review. Int J Pediatr Otorhinolaryngol. 2010;74(5):547-52. [Google Scholar] [Crossref]
- Mohan RP, Verma S, Agarwal N, et al. Central giant cell granuloma: a case report. Case Rep. 2013 Jul 20;2013: 903. [Google Scholar] [Crossref]
- Ahmed A, Naidu A. Towards better understanding of giant cell granulomas of the oral cavity. J Clin Pathol. 2021;74(8):483-90. [Google Scholar] [Crossref]
- Ramesh V. “Central giant cell granuloma”–An update. J Oral Maxillofac Pathol: JOMFP. 2020;24(3):413. [Google Scholar] [Crossref]
- Hillerup S, Hjørting-Hansen E. Aneurysmal bone cyst—simple bone cyst, two aspects of the same pathologic entity? Int J Oral Surg 1978 Feb 1;7(1):16-22. [Google Scholar] [Crossref]
- Richardson J, Stanbouly D, Litman E, et al. Central giant cell granuloma of the head & neck: A case report and systematic review. J Stomatol. Oral Maxillofac Surg.2021. [Google Scholar] [Crossref]
- Kerdoud O, Aloua R, Slimani F. Central Giant cell tumor of jaw bone in child: A case report. Int J Surg Case Rep. 2021;79:97-100. [Google Scholar] [Crossref]
- Pogrel MA. Calcitonin therapy for central giant cell granuloma. J Oral Maxillofac Surg. 2003;61(6):649-53. [Google Scholar] [Crossref]
- Tecco S, Caruso S, Nota A, et al. Bilateral Central Giant Cell Granuloma of the mandibular angle in three females from the same family. Head Face Med. 2018;14(1):1-8. [Google Scholar] [Crossref]
- Camarini C, de Souza Tolentino E. Non-surgical treatment as an alternative for the management of central giant cell granuloma: a systematic review. Clin Oral Investig 2021 Oct 1:1-22. [Google Scholar] [Crossref]
- Padmavathi Devi C, Swaroopkanth T, Sudhakar G, et al. Central giant cell granuloma of maxilla: A Case Report. Indian J Otolaryngol. Head Neck Surg. 2013;65(1):192-4. [Google Scholar] [Crossref]
- Chrcanovic BR, Gomez RS, Freire-Maia B. Neurofibromatosis type 1 associated with bilateral central giant cell granuloma of the mandible. J Cranio-maxillofac. Surg 2011;39(7):538-43. [Google Scholar] [Crossref]
- Flanagan AM, Speight PM. Giant cell lesions of the craniofacial bones. Head Neck Pathol. 2014;8(4):445-53. [Google Scholar] [Crossref]
- Edwards PC. Insight into the pathogenesis and nature of central giant cell lesions of the jaws. Med Oral Patol. Oral Cir Bucal. 2015;20(2):e196. [Google Scholar] [Crossref]
- Moghadam SA, Ghorbanpour M. Evaluation of Cyclin D1 Expression in Aggressive and Nonaggressive Central Giant Cell Granuloma of the Jaws. J Dent 2018;19(4):253. [Google Scholar] [Crossref]
- Jeyaraj P. Management of central giant cell granulomas of the jaws: an unusual case report with critical appraisal of existing literature. Ann Maxillofac Surg. 2019;9(1):37. [Google Scholar] [Crossref]
- Gomes CC, Diniz MG, Bastos VC, et al. Making sense of giant cell lesions of the jaws (GCLJ): lessons learned from next‐generation sequencing. J Pathol.2020;250(2):126-33. [Google Scholar] [Crossref]
- Kaban LB, Dodson TB. Regarding “Denosumab for the management of central giant cell granuloma of the jaws—a case series”. Int J Oral Maxillofac Surg.2022 Jun 1;51(6):844-5. [Google Scholar] [Crossref]
- de Lange J, van den Akker HP, van den Berg H. Central giant cell granuloma of the jaw: a review of the literature with emphasis on therapy options. Oral Surg Oral Med. Oral Pathol Oral Radiol Endodontology. 2007;104(5):603-15. [Google Scholar] [Crossref]
- Bayar OF, Ak G. Treatment of giant cell granuloma with intralesional corticosteroid injections: a case report. J Istanb Univ Fac Dent. 2015;49(3):45. [Google Scholar] [Crossref]