N-Nitrosation of dimethylamine by Bacillus cereus isolated from fermented Palm sap (Elaeis guineensis)
2 Department of Biological Sciences, Biochemistry Unit, Covenant University, Ota, Ogun State, Nigeria
Received: 26-Dec-2017 Accepted Date: Jan 05, 2018; Published: 12-Jan-2018
Citation: Gbadebo A, Adedosu OT, Oyewo EB, et al. N-Nitrosation of dimethylamine by Bacillus cereus isolated from fermented Palm sap (Elaeis guineensis). J Food Clin Nutr 2017;1(1):5-11.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
This study investigated the nitrosating activities of intact cells and cell fractions of Bacillus cereus isolated from fermented palm wine [Elaeis guineensis] during incubation with dimethylamine and nitrite or nitrate. The pH values of the incubation mixtures were 7.2 ± 0.07 and 7.1 ± 0.07 in the presence of nitrite and nitrate, respectively. The N-Nitrosodimethylamine [NDMA] level in cell debris was significantly higher compared with cell extract, cell suspension and sterile controls. The time-course of NDMA formation in cell debris showed a significant [p<0.05] early decline followed by an increase. The kinetic data obtained from Lineweaver-Burk plots of NDMA formation showed that Michaelis -Menten constant [Km] value was 37.5% lower, while initial velocity [Vmax] was 20.0% higher in cell debris relative to cell extract, when a fixed DMA concentration was used against varying nitrite concentrations. However, at a fixed nitrite concentration against varying DMA concentration, Km value was 44.4% lower, while Vmax was 166.7% higher in cell debris compared with cell extract. Investigation of nitrosation mechanism showed that Vmax values in the cell debris were 41.7, 50.0 and 55.6 μmol NDMA/mg protein, while the values were 34.5, 37.0 and 43.5 μmol NDMA/mg protein in cell extract for the respective three nitrite concentrations.
This study has shown that intact Bacillus cereus cells catalyzed nitrosation of dimethylamine at near neutral pH, and the nitrosation, which was higher in cell debris than cell extract of the bacterium, followed a sequential mechanism of enzyme catalysis.
Introduction
Palm wine is a whitish beverage obtained from the sap of palms, such as Elaeis guineensis and Raphia hookeri, commonly found in the tropical region [1,2]. The sap naturally undergoes fermentation after tapping from the palms due to contamination by certain bacteria and yeast from the tapping environment and even instruments [3,4]. Their studies were later supported by several studies reporting the isolation of microbes including Bacillus, Lactobacillus, Brevibacterium, Staphylococcus, Micrococcus, Escherichia and Saccharomyces [5], Leuconostoc palmae spp. [6] and Saccharomyces spp., Bacillus cereus, Bacillus firmus and Enterococcus feacalis in palm wine [7] from fermented palm wine. In the recent time, our research group isolated Acetobacter aceti from fermented palm wine of the E. guineensis brand [8]. Bacillus cereus is a rod-shaped bacterium, which is Gram-positive, flagellated and spore-forming in nature [9]. This saprophytic bacterial species is commonly present in the soil environment and as a microbial contaminant in several foods. Being present in foods makes it possible for this bacterium to be ingested, therefore becoming part of the microflora in the intestinal tract of humans [10,11]. Isolates of this bacterium from foods and human gut can be subdivided into mesophilic and psychrotrophic strains. The former strain thrives at 37°C, while the latter thrives under refrigeration condition [12]. B. cereus-induced food poisoning can either be diarrhoeal type or emetic type. The diarrhoeal type occurs when foods contaminated with large amount of the bacterial cells or spores are ingested, while the emetic type is associated with ingestion of foods containing the pre-formed toxin of the bacterium. The growth of this bacterial species is optimal in the presence of oxygen, but can also occur under anaerobic conditions [13]. The formation of N-nitrosamines from amines or amine-related compounds in the presence of nitrite or nitrate has been well documented through several studies, as a complex process involving both enzymatic and chemical mechanisms [8,14-16]. Cultures of Escherichia coli, Bifidobacterium, Pseudomonas stutzeri, Cryptococcus terreus, Xanthomonas campetris and several other bacteria have been found to catalyze the formation of N-nitrosodimethylamine [NDMA] [15,17]. The abilities of some bacteria to convert trimethylamine to dimethylamine and reduce nitrate to nitrite with the subsequent reaction of the two products to form dimethylnitrosamine have also been reported by Ayanaba and Alexander [18]. It has however been shown that the activities of bacteria in catalyzing endogenous nitrosation from nitrate or nitrite and secondary amines could be associated with the etiology of several cancers, such as gastric cancer [19,20]. The direct activity of bacteria in amine nitrosation has been established and found to be linked to the presence of nitrate reductase enzyme in the organisms [21,22]. A number of microorganisms have been observed to produce N-nitrosodiphenylamine from diphenylamine and nitrite at pH 7.5, and the soluble enzymes catalyzing the N-nitrosation of the compound were obtained from two of these organisms [18]. Soluble fractions obtained from the cell suspensions of certain bacteria and yeast, isolated from fermented palm wine, catalyzed nitrosation at neutral pH, when incubated with diphenylamine [DPA], diethylamine [DEA] or dimethylamine [DMA] in the presence of nitrite and glucose. The intrinsic factor in the soluble extracts of the microorganisms responsible for the nitrosation was then suggested to be a heat-labile factor, suggested to be an enzyme [23]. The objectives of the present study were to investigate the nitrosating activity of Bacillus cereus, isolated from fermented palm wine, as well as the kinetics and possible mechanism involved during incubation of this bacterium with dimethylamine and nitrite or nitrate.
Materials and Methods
Dimethylamine [DMA] was purchased from Sigma-Aldrich, U.S.A. Fresh Palm wine was bought from a Palm wine tapper in Ogbomoso, Oyo State, Nigeria. All other chemicals were of analytical grades.
Identification, isolation and re-culturing of Bacillus cereus cells
Fresh palm wine [Elaeis guineensis] was bought from Obamoro village near Iwo town [Osun State] and put in a pre-sterilized 100 mL bottle. An aliquot [1.0 mL] of palm wine was taken aseptically after 72 hours of fermentation. The sample was serially diluted 10-fold in 0.1% peptone water, and 1.0 ml of diluted sample was plated out in duplicate using spread plate method [24] on tryptone soy agar [25]. The plates were aseptically incubated at 30°C for 24 hours. The bacterial cells were morphologically identified and then re-isolated in 50 mL of peptone water medium to obtain a pure culture as previously described by Njoku et al. [26].
Determination of pH, microbial growth and time-course of nitrosation in a culture of Bacillus cereus cells incubated with dimethylamine and nitrite or nitrate
The bacterial cells [2.0 mL of pure culture] were incubated [in duplicates] in 50 mL of 0.1% peptone water medium containing 1.0 mL of 25 mM dimethylamine and 1.0 mL of 10 mM sodium nitrite [NaNO2] or 10 mM sodium nitrate [NaNO3] for 5 days inside 100 mL Erlenmeyer flasks, kept in a sterile condition in a hood maintained at 37°C. The incubation flasks were subjected to constant shaking to ensure homogenous air circulation, temperature and nutrients for the growing cells. Sterile controls, which were run in parallel to the bacterial cultures, contained dimethylamine, nitrite or nitrate and 0.01 M phosphate buffer [used in place of microbial suspension] in peptone water medium. The growth of the bacterial cells was monitored by measuring the optical density [absorbance] of the cell suspension at 600 nm on daily basis. The pH of the cell suspension was also determined daily using pH meter just before addition of sulfamic acid. On daily basis, an aliquot of 10 mL of cell suspension was aseptically collected from the incubation flasks and treated with 3 mL of 10% sulfamic acid to quench nitrosation by decomposing excess nitrite [27]. The cells were harvested by centrifugation at 10000 x g for 10 minutes. The pellets [bacterial cells] were washed in phosphate buffer [pH 7.2] and resuspended in the same buffer. The bacterial cells were homogenized using glass beads [diameter 0.045 mm] in a cell homogenizer for 5 minutes [28]. The crushed cells were centrifuged at 10000 x g for 10 minutes to remove the glass beads with the unbroken cells. The supernatant was further centrifuged at 37,000 x g [refrigerated centrifuge] for 1 hour to obtain cell extract and cell debris, which were kept at 40c until use. The N-nitrosodimethylamine [NDMA] concentrations of sterile control, cell suspension, cell extract and cell debris were determined spectrophotometrically according to Montgomery and Dymock [29]. Briefly, 1.0 mL of the test sample was treated with 0.5 mL of 0.5% sodium carbonate in a covered test tube and then exposed to ultra-violet radiation for 15 minutes. The mixture was sequentially treated with 1.5 mL each of sulphanilic acid and Naphthylethylene diamine solutions with shaking. The absorbance of pink solution was read at 550 nm. The protein concentrations were estimated by the method of Lowry et al. [30].
Fractionation of intact Bacillus cereus cells
Intact bacterial cells [2.0 mL of pure culture] were incubated in 50 mL of 0.1% peptone water medium for 5 days, and then harvested by centrifugation at 10,000 x g for 10 minutes. The incubation mixture was contained in a 100 mL Erlenmeyer flask. The pellets [Bacillus cereus cells] were suspended in 0.01 M Phosphate buffer [pH 7.2] and then homogenized using glass beads [diameter 0.045 mm] in a cell homogenizer for 5 minutes [28]. The crushed cells were centrifuged at 10000 x g for 10 minutes to remove the glass beads with the unbroken [intact] cells. The supernatant was subjected to further centrifugation at 37,000 x g [refrigerated centrifuge] for 1 hour to obtain cell extract and cell debris, which were kept at 4°C until use.
Determination of the Km and Vmax of nitrosation by cell extract and cell debris incubated with varying concentrations of dimethylamine and a fixed concentration of sodium nitrite
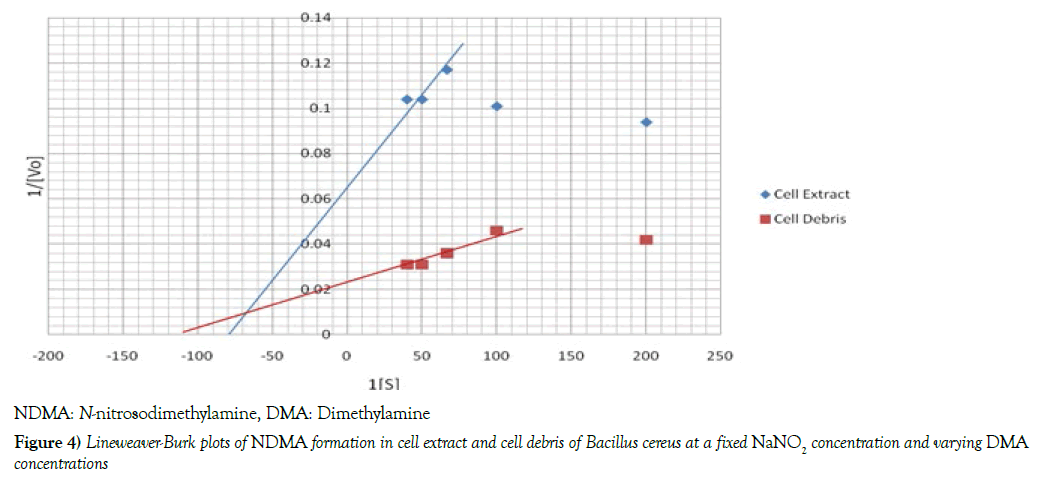
Cell extract and cell debris [2.0 mL] each was incubated in a 50 mL Erlenmeyer flask with total volume of 20 mL reaction mixture [phosphate buffer, 0.01 M, pH=7.2] containing 1 mL of dimethylamine solution [5 mM, 10 mM, 15 mM, 20 mM, 25 mM] and 1.0 mL of 25 mM sodium nitrite solution at 37°C for 15 minutes. Nitrosation was quenched by gradually adding 3.0 mL of 10 % Sulfamic acid [27]. The protein concentrations of test samples were estimated by the method of Lowry et al. [30]. The concentrations of N-nitrosodimethylamine [NDMA] formed were spectrophotometrically determined according to Montgomery and Dymock, [29] as initial velocities. Briefly, 1.0 mL of the test sample was mixed with 0.5 mL of 0.5% sodium carbonate [to trap the nitroso group released by irradiation] in a covered test tube and then exposed to ultra-violet radiation for 15 minutes. The irradiated mixture was sequentially treated with 1.5 mL each of sulphanilic acid and Naphthylethylene diamine [NEDA] solutions with shaking. After 15 minutes, the absorbance of the pink solution was read at 550 nm. Lineweaver-Burk [double reciprocal] plot of initial velocities against varying dimethylamine concentrations was obtained and the Michaelis-Menten constant [Km] and maximum velocity [Vmax] values were determined.
Determination of the Km and Vmax of nitrosation by cell extract and cell debris during incubation with varying concentrations of sodium nitrite and a fixed concentration of dimethylamine
Cell extract and cell debris [2.0 mL] each was incubated in a 50 mL Erlenmeyer flask with a total volume of 20 mL reaction mixture [phosphate buffer, 0.01 M, pH 7.2] containing 1 mL of sodium nitrite solution [5 mM, 10 mM, 15 mM, 20 mM and 25 mM] and 1 mL of 25 mM dimethylamine solution at 37°C for 15 minutes. Nitrosation was quenched by gradually adding 3.0 mL of 10 % Sulfamic acid [27]. The protein concentrations of test samples were estimated by the method of Lowry et al. [30]. The concentrations of N-nitrosodimethylamine [NDMA] formed were spectrophotometrically determined [29] as initial velocities. Briefly, 1.0 mL of the test sample was mixed with 0.5 mL of 0.5% sodium carbonate [to trap the nitroso group released by irradiation] in a covered test tube and then exposed to ultra-violet radiation for 15 minutes. The irradiated mixture was sequentially treated with 1.5 mL each of sulphanilic acid and Naphthylethylene diamine [NEDA] solutions with shaking. After 15 minutes, the absorbance of the pink solution was read at 550 nm. Lineweaver-Burk [double reciprocal] plot of initial velocities against varying dimethylamine concentrations was obtained and the Michaelis-Menten constant [Km] and maximum velocity [Vmax] values were determined.
Determinations of the mechanism of nitrosation by cell extract and cell debris of Bacillus cereus incubated with dimethylamine and different concentrations of sodium nitrite
Each of cell debris and cell extract [2.0 mL] was incubated in a 50 mL Erlenmeyer flask with a total volume of 20 mL reaction mixture [phosphate buffer, 0.01 M, pH 7.2] containing 1.0 mL of dimethylamine solutions [5 mM, 10 mM, 15 mM, 20 mM and 25 mM] and 1.0 mL of sodium nitrite solutions at three different concentrations [15 mM, 25 mM, 50 mM] at 37°C for 15 minutes. Nitrosation was quenched by gradually adding 3 mL of 10% Sulfamic acid according to Kunisaki and Hayashi [27]. The protein concentrations of test samples were determined using the method of Lowry et al [30]. The concentrations of N-nitrosodimethylamine [NDMA] formed during incubation were spectrophotometrically determined [29] as initial velocities. Briefly, 1.0 mL of reaction mixture was mixed with 0.5 mL of 0.5% sodium carbonate [to trap the N-nitroso group released by irradiation] in a covered test tube and then exposed to ultra-violet radiation for 15 minutes. The irradiated mixture was sequentially treated with 1.5 mL each of sulphanilic acid and Naphthylethylene diamine [NEDA] solutions with shaking. After 15 minutes, the absorbance of the pink solution was read at 550 nm. Lineweaver-Burk [double reciprocal] plots of initial velocities against varying dimethylamine concentrations were obtained for the three different concentrations of sodium nitrite, and the Michaelis-Menten constant [Km] and maximum velocity [Vmax] values were determined.
Statistical analysis
Data were expressed as mean ± standard deviation. Student-t was performed to determine the significance at 95% confidence level, and statistically significant values were taken at p<0.05.
Results
The pH, microbial growth and Time-course of nitrosation
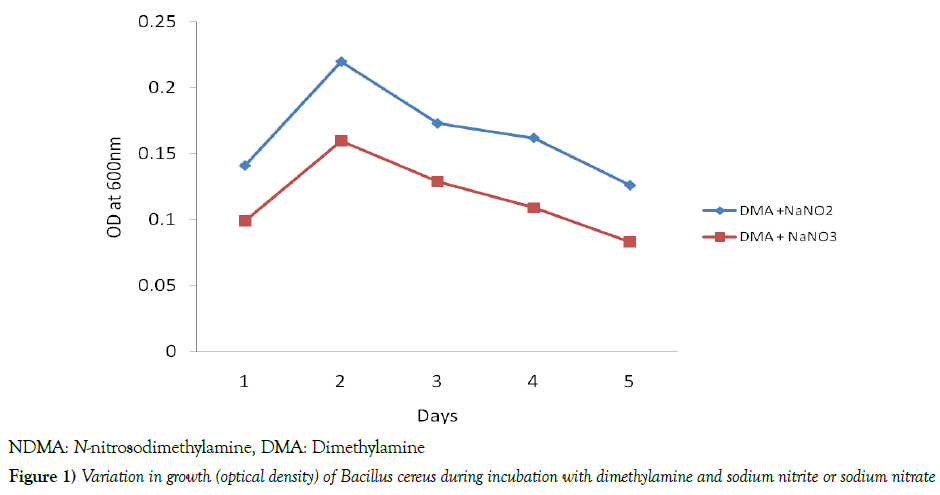
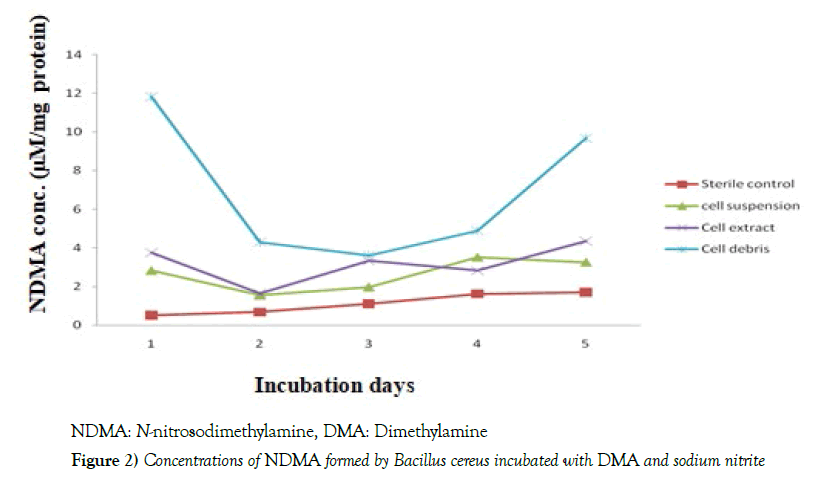
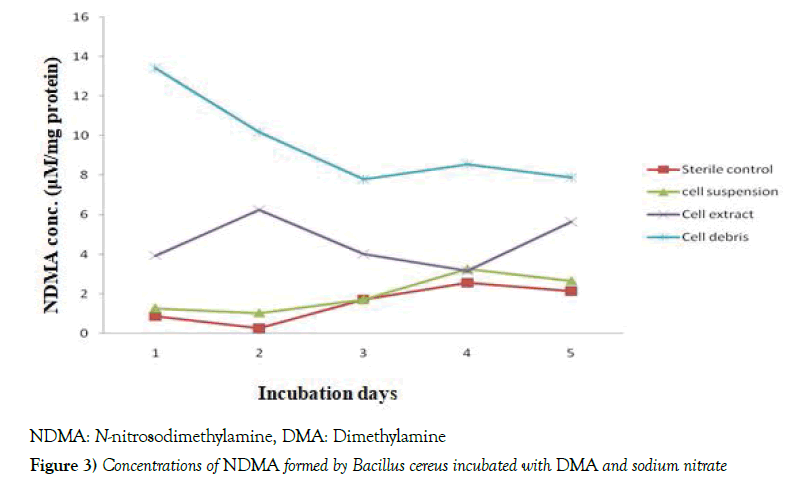
The results in Table 1 depicted that the pH values of Bacillus cereus cells suspension during the incubation at 37 0C with DMA were found within the ranges of 7.1 - 7.3 [7.2 ± 0.07] and 7.0 – 7.2 [7.1 ± 0.07] in the presence of nitrite and nitrate, respectively. When the growth of the bacterium was determined against the days of incubation, it was noticed that optimum growth of the bacterium occurred on day 2, followed by a significant decline [p<0.05] in both the nitrite- and nitrate-grown cultures, throughout the remaining period of incubation. However, the growth of the bacterium was significantly [p<0.05] higher in the nitrite-grown than the nitrate-grown culture throughout the period of incubation, as shown in Figure 1. The data in Table 2 indicated that in the nitrite-grown cell cultures, as from day 2, the optical density [microbial growth] at 600 nm was observed to be higher than day 1, however, only days 2 and 3 recorded significant [p<0.05] values compared with day 1. The highest concentration of NDMA formed by the bacterium was noticed on day 1, after which there were decreases in the levels of NDMA from day 2, but significant [p<0.05] values were recorded only on days 2, 3 and 4 relative to day 1. Still on Table 1, in the nitrate-grown cultures, significant [p<0.05] increases were noticed from day 2 to 5 compared with day 1. In the same nitrate-grown cultures, however, only days 3 and 4 recorded significant [p<0.05] reduction compared with day 1. Figures 2 and 3 depicted the time-course of NDMA formation in the sterile control, cell suspension, cell extract and cell debris of Bacillus cereus during incubation of intact cells with dimethylamine and nitrite or nitrate. In Figure 2, when nitrite was used, NDMA level in the sterile control was found to steadily increase during the incubation, however, the level was significantly [p<0.05] lower than was observed in each of the cell suspension, cell extract and cell debris, with cell debris showing the highest level. There were almost comparable trends in the time-course of NDMA formation between cell extract and cell suspension. However, in the cell debris, NDMA formation significantly [p<0.05] declined in the early period of incubation, and thereafter began to rise. In Figure 3, when nitrate was used, there were comparable trends in the time-course of NDMA formation between sterile control and cell suspension, in which an initial decrease was observed, followed by a significant [p<0.05] increase during the remaining period of incubation. In the cell extract, the level of NDMA increased significantly [p<0.05] in the early part of the incubation and declined significantly [p<0.05] for 2 days [day 2 to day 4], followed by another turn of increment. However, in cell debris, NDMA level significantly [p<0.05] declined in the early period of incubation [day 1 to day 3] and began to slightly level up in the course of further incubation as also shown in Figure 3.
Table 1: Change in pH of cell suspension of Bacillus cereus incubated with dimethylamine and sodium nitrite or sodium nitrate
| pH | ||
|---|---|---|
| Treatments | DMA + NaNO2 | DMA + NaNO3 |
| Day 1 | 7.1 + 0.0 | 7.0 + 0.4 |
| Day 2 | 7.2 + 0.1 | 7.1 + 0.0 |
| Day 3 | 7.2 + 0.0 | 7.1 + 0.2 |
| Day 4 | 7.3 + 0.4 | 7.1 + 0.0 |
| Day 5 | 7.2 + 0.2 | 7.2 + 0.1 |
| Mean ± S.D | 7.2 ± 0.07 | 7.1 ± 0.07 |
Data expressed in Mean ± S.D, n=2; DMA: Dimethylamine, NaNO2: Sodium nitrite, NaNO3: Sodium nitrate
Table 2: Relative variations in Bacillus cereus growth (Optical density) and concentrations of NDMA formed during incubation with DMA and nitrite or nitrate
| Incubation Days | Optical density at 600nm with nitrite | NDMA (µM/mg protein) with nitrite | Optical density at 600nm with nitrate | NDMA (µM/mg protein) with nitrate |
|---|---|---|---|---|
| 1 | 0.131 | 18.39 | 0.099 | 18.65 |
| 2 | 0.218# | 7.45# | 0.170# | 17.46 |
| 3 | 0.163# | 8.90# | 0.144# | 13.51# |
| 4 | 0.148 | 11.21# | 0.159# | 14.97# |
| 5 | 0.122 | 17.28 | 0.129# | 16.17 |
NDMA: N-nitrosodimethylamine, DMA: Dimethylamin; # statistically different compared with day 1
Kinetic studies
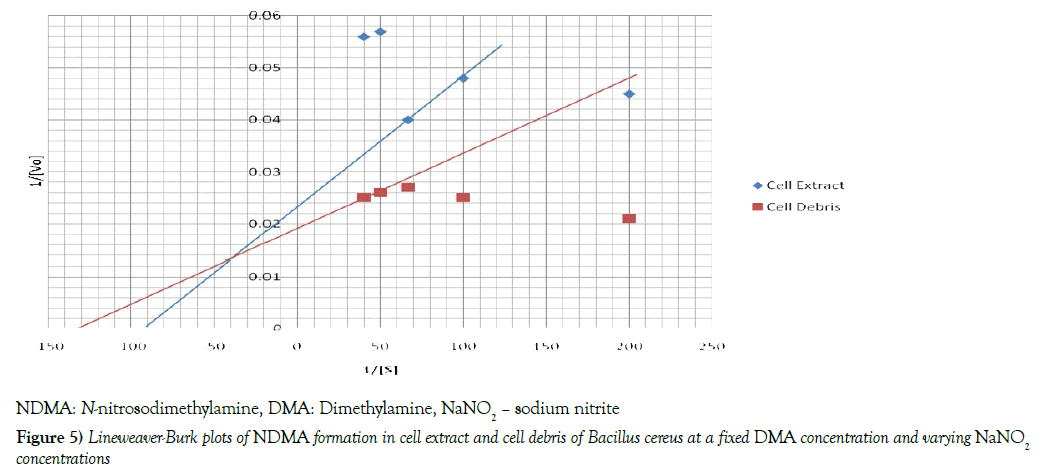
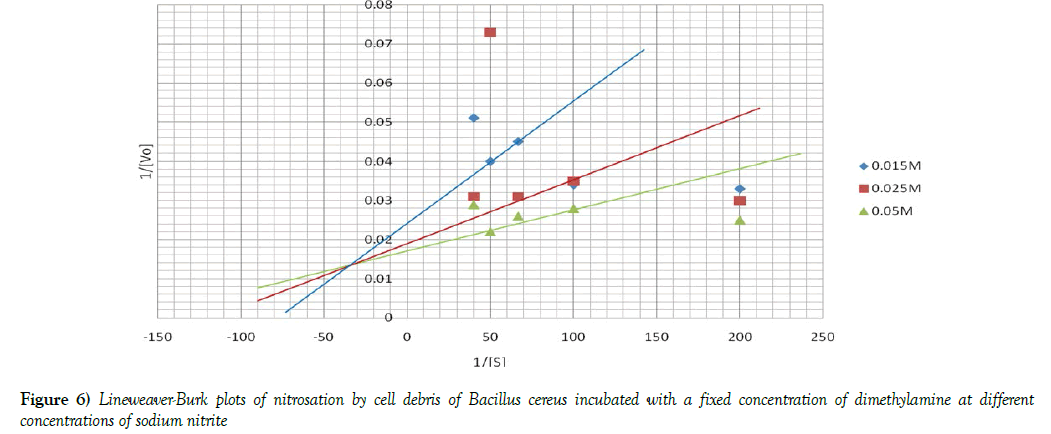
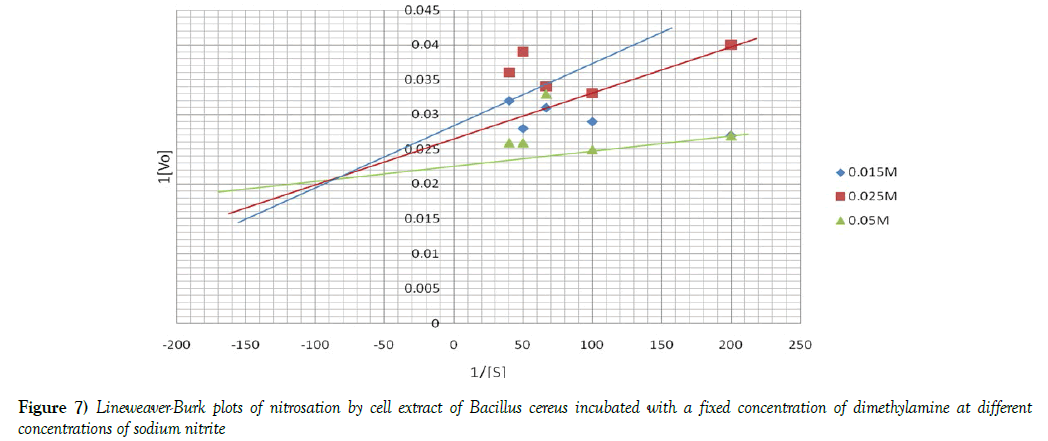
The Michaelis -Menten constant [Km] and initial velocities [Vmax] of nitrosation in both cell debris and cell extract were determined at a fixed concentration of DMA and varying concentrations of sodium nitrite and vice-versa. To determine these two parameters, the reciprocal of initial velocities [1/V] were primarily plotted against the corresponding reciprocal of the substrate [1/S] in each incubation system to obtain Lineweaver-Burk plots or double reciprocal plots, as shown in Figures 4 and 5. The Km and Vmax values were calculated and the results summarized in Table 3. When a fixed DMA concentration was used against varying concentrations of nitrite, the Km value was 37.5% lower, while Vmax was 20.0% higher in cell debris compared with cell extract. However, when a fixed nitrite concentration was used against varying concentrations of DMA, the Km value was 44.4 % lower, while Vmax was 166.7 % higher in cell debris compared with cell extract. Furthermore, we investigated the possible mechanism of nitrosation in both cell debris and cell extract of the bacterium, and the results are presented as double reciprocal plots (Figures 6 and 7). The Lineweaver-Burk plots presented the clockwise shifting of the three lines as the concentration of nitrite increased, all meeting at a point above the horizontal [1/S] axis. The Vmax values in the cell debris were observed to be 41.7, 50.0 and 55.6 μmol NDMA/mg protein for the respective three concentrations of nitrite [0.015, 0.025 and 0.05 mM]. However, in the cell extract, the Vmax values were calculated to be 34.5, 37.0 and 43.5 μmol NDMA/mg protein for the respective three concentrations of nitrite [0.015, 0.025 and 0.05 mM]. It is noteworthy that the clockwise shifting of the three lines corresponded with increase in the Vmax during NDMA formation in both cell debris and cell extract.
Table 3: Values of Km and Vmax of nitrosation by cell debris and cell extract of Bacillus cereus incubated with dimethylamine and sodium nitrite
| Cell fractions | Fixed DMA + Varying nitrite | Fixed nitrite + Varying DMA | ||
|---|---|---|---|---|
| Km (μmol NDMA) | Vmax (μmol NDMA/mg protein) | Km (μmol NDMA) | Vmax (μmol NDMA/mg protein) | |
| Cell debris | 0.008 | 50 | 0.009 | 41.67 |
| Cell extract | 0.011# | 41.67## | 0.013# | 15.63## |
Km –Michaelis-Menten constant; Vmax – Maximum velocity; DMA- dimethylamine; NDMA – N-nitrosodimethylamine; # Km statistically higher compared with cell debris; ## Vmax statistically lower compared with cell debris
Discussion
In view of the toxicological importance of N-nitrosodimethylamine [NDMA] and the ubiquitous presence of bacteria in nature, this study was aimed at investigating the condition and kinetics of formation of this N-nitrosamine by Bacillus cereus isolated from a widely consumed palm beverage of Elaeis guineensis.
The present study noticed that the intact cells of Bacillus cereus catalysed the nitrosation of dimethylamine within a narrow range of pH values found to be near neutral in both the nitrite-grown [7.2 ± 0.07] and nitrategrown [7.1 ± 0.07] cultures. Several works have studied the pH condition during microbial formation of N-nitrosamines. Soluble enzymes obtained from certain microorganisms have been observed to catalyse the synthesis of N-nitrosodiphenylamine from diphenylamine and nitrite at pH 7.5, according to Ayanaba and Alexander [18]. In a study carried out by Kunisaki and Hyashi [27], they reported that resting cells of Escherichia coli formed N-nitrosamines at an optimum pH of 8.0, whereas an optimum pH of 3.4 was noticed during chemical nitrosation. Certain microbiological studies revealed that optimum growth of B. cereus occurred at a pH range of 4.9- 10.0 [31,32]. However, Shukor et al. [33] reported a pH range of 6.8-7.0 for the optimum growth of this bacterium. The formation of NDMA by B. cereus at near neutral pH condition, as observed in the present study, may implicate enzymatic involvement in the NDMA synthesis. This observation is in line with certain studies by Kunisaki and Hyashi [27], Mills and Alexander [34] and Kunisaki et al. [35], who reported that microbial nitrosation could involve some enzymatic mechanisms inherent in the microbial cells. During the incubation with NDMA precursors, bacterial cell growth was turbidimetrically [optical density at 600 nm] determined against the incubation period. The growth of B. cereus during the incubation significantly increased in the early period, and later declined in both nitriteand nitrate-grown cultures till the end of incubation. However, a comparison of the bacterial growth between the two cultures showed a higher intensity in the nitrite-grown than nitrate-grown culture. The higher growth intensity in the nitrite-grown cells than the nitrate-grown cells may suggest a greater ability of nitrite to induce cell growth than nitrate.
Studying the relative variations between the growth of B. cereus and concentrations of NDMA formed in the cell cultures by the intact cells, the initial sharp increase in growth with corresponding decreases in NDMA concentration in both nitrite- and nitrate- grown cultures, which was later accompanied by a general rise in NDMA level, when the growth declined, suggested that the bacterial cells could potentially synthesise NDMA from its precursors and as well utilize this compound for some growth. The utilization of NDMA by bacteria as a sole source of carbon has not been reported, however, bacterial cells could potentially utilize NDMA as a sole source of nitrogen, resulting in formaldehyde formation [36]. In Rhodococcus species, reducing equivalents and fixable carbon are generated from formaldehyde for the use of the bacterium [37]. The ability of a microorganism to utilize an environmental toxicant, thereby reducing its level to a nontoxic status is an important approach to environmental bioremediation [38].
The intact cells of B. cereus were incubated with NDMA precursors [being run parallel with sterile controls] for 5 days, and then centrifuged, followed by cell fractionation. In the nitrite –grown cells, the time-courses in sterile controls, cell extract and cell suspension showed a nearly similar trend in NDMA formation. However, the time-course of NDMA formation in the cell debris exhibited an initial decrease, and later began to rise. NDMA concentration in the sterile control was significantly lower than what was obtained in each of the cell suspension, cell extract and cell debris, with cell debris showing the highest level. In the nitrate-grown cells, comparable trends were noticed in the time-course of NDMA formation between sterile control and cell suspension. The cell extract, showed an inconsistent timecourse of NDMA formation in the course of incubation, while cell debris, exhibited an early significant decline, which later slightly leveled up in the course of further incubation. Overall, there was a significantly higher NDMA formation, showing a faster rate, in the intact cells compared with sterile controls in both nitrite- and nitrate-grown cells. This significant difference noticed the time-course of NDMA formation could be an indication of enzymatic involvement in the bacterial nitrosation. Comparing the NDMA levels in both cell debris and cell extract of the bacterium, we noticed a significantly higher level in the cell debris relative to cell extract, which may indicate a higher degree of nitrosation in the cell debris than cell extract. We have earlier reported similar observations in our previous study using Acetobacter aceti, also isolated from fermented palm wine [8].
The mechanism of nitrosation by denitrifying bacteria has been reported to vary from one organism to the other. For instance, Calmels et al. [22] studied the effects of different culture conditions on the nitrosating abilities of Paracoccus denitrificans, Escherichia coli, Proteus morganii and Pseudomonas aeruginosa, and reported that the nitrosating abilities of the first three organisms correlated with their abilities to reduce nitrate to nitrite before NDMA formation, whereas nitrosation by the fourth organism was strongly induced in a minimum medium containing either nitrite or nitrate. It has been suggested that nitrosation occurs via the activity of a nitrate reductase in the presence of nitrate, while a nitrite reductase catalyses nitrosation in the presence of nitrite [22,39]. The data from the present study therefore suggests that the activity of nitrate reductase is higher than that of nitrite reductase in B. cereus, as reflected from the higher level of NDMA in the nitrate-grown relative to nitrite-grown cultures. A comparison of NDMA formations between the cell extract and cell debris shows a significantly higher level in cell debris than cell extract. The higher activity of the nitrosating enzymes in the cell debris therefore suggests a higher activity of these enzymes in the membrane and periplasm than the cytosol of the bacterium. A similar observation was also reported from our previous study, showing a higher level of NDMA in the cell debris than cell extract of Acetobacter aceti [8]. This is also consistent with a study by Bedzyk et al. [40] who demonstrated that bacterial membrane-bound and periplasmic nitrate reductases play important roles in dissimilatory nitrate reduction leading to nitrosamines formation.
The kinetic data of NDMA formation obtained from this study were consistent with those of enzyme-catalyzed reactions. The Km and Vmax of nitrosation in both cell debris and cell extract were determined at a fixed concentration of DMA and varying concentrations of sodium nitrite and vice-versa. In both cases, the Km values of nitrosation were found to be significantly lower in cell debris than cell extract, while the Vmax values were significantly higher in cell debris than cell extract. The lower Km and higher Vmax in cell debris than cell extract indicate that nitrosation has occurred at a higher degree in cell debris than cell extract in B. cereus, suggesting a higher activity of the nitrosating enzymes in the cell membrane than cytosol of the bacterium. An earlier observation of higher concentration of NDMA in the cell debris than cell extract of the intact cells made in the present is a further support. In line with these present observations, we have earlier reported a higher degree of nitrosation in the cell debris than cell extract of Acetobacter aceti [8]. Nitrate reductase enzymes, involved in nitrosation, are located in the bacterial membrane and periplasmic region, which constitute the major constituents of microbial cell debris fraction [40,41]. Since nitrosation is a bisubstrate reaction, this study investigated the type of mechanism involved in the nitrosation by cell debris and cell extract of B. cereus. The data from this study show that the Lineweaver-Burk plots demonstrated clockwise shifting of the three lines as the concentration of nitrite increased, and they met at a point above the horizontal [1/S] axis. More importantly, the clockwise shifting of the three lines corresponded with increase in the Vmax during NDMA formation in both cell debris and cell extract. A similar data were recorded from our previous study using Acetobacter aceti in a nitrosation of dimethylamine with nitrite [8]. The type of shifting observed in the present study has been described by Cleland [42,43] to characterize sequential mechanism of bisubstrate enzyme catalysis. According to Palmer [44], it could be suggested that this bacterial nitrosation may involve formation of a ternary complex in the course of the catalysis.
Conclusion
From the data shown in the present study, it could be inferred that intact Bacillus cereus cells catalyzed the nitrosation of dimethylamine optimally at near neutral pH, and that cell debris showed a higher degree of nitrosation than cell extract. In addition, the mechanism of the bacterial nitrosation showed a sequential pattern of enzyme catalysis.
Acknowledgement
Mr Oladapo M.O. of the Microbiology Unit, Institute of Agricultural Research and Training [IAR &T] Ibadan, Oyo State, Nigeria, for the isolation of the Bacillus cereus cells from fermented Palm wine.
REFERENCES
- Uzogara SG, Agu LN, Uzogara EO. A review of traditional fermented food condiments and beverages in Nigeria: Their benefits and possible problems. Ecology of Food and Nutrition 1990;24:267-288.
- Uzochukwuru BUA, Balogh FE, Ngoddy PD. Standard pure culture inoculum of natural fermented palm sap. Nig J Microbiol 1991;9:67-77.
- Okafor N. Palm wine yeast from parts of Nigeria. J Sci Food Agric 1987;23:1399-1407.
- Faparusi SI, Basir O. Factors affecting the qualities of palm wine. W Afr J Biol Appl Chem 1991;15:18-28.
- Ogbulie TE, Ogbulie JN, Njoku HO. Comparative study on the microbiology and shelf life stability of palm wine from Elaeis guineensis and Raphia hookeri obtained from Okigwe, Nigeria. Afr J Biotechnol 2007;6:914-922.
- Ehrmann MA, Freidling S, Vogel RF. Leuconostoc palmae spp. nov: A novel lactic acid bacterium isolated from palm wine. Int J Syst Evol Microbiol 2009;59:943-947.
- Olawale AK, Akintobi AO, David MO. Evaluation of microbial quality and Alcoholic improvement of natural and fermented Raphia palm wine (0goro). New York Science Journal 2010;3:2.
- Adeleke GE, Akpabio CJ, Oyewo EB, et al. Acetobacter aceti isolated from fermented palm wine in the South west region of Nigeria elucidated high nitrosamine production in the presence of nitrite. J Toxicol and Health 2015;106: 494-502.
- Montville TJ, Matthews KR. Food Microbiology: An Introduction. Second edition, ASM Press, Washington D.C. 2005.
- Jensen GB, Hansen BM, Eilenberg J, et al. The hidden lifestyles of Bacillus ceresus and relatives. Environmental Microbiology 2003;5:631-640.
- Vilain S, Luo Y, Hildreth M, et al. Analysis of the life cycle of the soil saprophyte Bacillus cereus in liquid soil extract and in soil. Appl Environ Microbiol 2006;72:4970-4977.
- Wijnands LM, Dufrenne JB, Zwietering MH, et al. FM Spores from mesophilic Bacillus cereus strains germinate better and grow faster in simulated gastro- intestinal conditions than spores from psychrotrophic strains. Int J Food Microbiol 2006;112:120-128.
- Mols M, Pier I, Zwietering MH, et al. The impact of oxygen availability on stress survival and radical formation of Bacillus cereus. Int J Food Microbiol 2009;135:303-311.
- Maduagwu EN, Joaqium KA, Bassir OO. Microbial nitrosamine formation in palm wine: In vitro N-nitrosation by cell suspensions. J Environ Pathol Toxicol 1979;2:1183-1194.
- Smith NA, Smith P, Woodruff CA. The role of Bacillus spp. in N-nitrosamine formation during wort production. J Inst Brew 1992;98: 409-414.
- Jang CM, Heinze TM, Deck J, et al. Transformation of N-Phenylpiperazine by mixed cultures from a Municipal wastewater treatment plant. Appl Environ Microbiol 2008;74:6147-6150.
- Atawodi SE. Occurrence of preformed volatile nitrosamines in preparations of some Nigerian medicinal plants: a preliminary report. Food Chem Toxicol 2003;41:551-554.
- Ayanaba A, Alexander M. Microbial formation of Nitrosamines In Vitro. Appl Microbiol 1973;25: 862-863.
- Jakszyn P, Gonzalez CA. Nitrosamine and related food intake and gastric and oesophageal cancer risk: a systematic review of the epidemiological evidence. World J Gastroenterol 2006;12:4296-4303.
- Boffeta P, Hecht S, Gray N, et al. Smokeless tobacco and cancer. Lancet Oncol 2008;9:667-675.
- Suzuki S, Mitsuoka T. N-nitrosamine formation by bacteria of the intestine. In N-nitroso compounds: Occurrence, Biological Effects and Relevance to Human Cancer (IARC Scientific Publications no. 57) In: IO O’Neill, RC von Borstel, et al. IARC 1984;275-281.
- Calmels S, Ohshia A, Bartsch H. Nitrosamine formation by Denitrifying and Non-denitrifying bacteria: Implication of nitrite reductase and nitrate reductase in nitrosation catalysis. J Gen Microbiol 1988;134:221-226.
- Joaqium K. Nitrosamine contamination of some Nigerian beverages. PhD Thesis, University of Ibadan, Nigeria. 1973.
- Cheesbrough M. Medical laboratory manual for Tropical countries. Volume 2, Microbiology. Butterworth, Heinemann Ltd. Cambridge 1994;56-58.
- Cruikshank R, Duiguid PJ, Mamon PC, et al. Medical Microbiology: The practice of medical Microbiology. Churchhill living stone, Edinburgh 1982;587.
- Njoku HO, Ogbulie JN, Nnubia O. Microbial Ecology of traditional fermentation of African oil bean seed for Ugba production. J Food Microbiol 1990;3:18-28.
- Kunisaki N, Hayashi M. Formation of secondary amines and nitrite by resting cells of Escherichia coli B. Appl Environ Microbiol 1979;37:279- 282.
- Venturin C, Zulaika J, Boze H, et al. Purification and properties of an Alcohol dehydrogenase (HUADHII) from Hanseniasipora uvarum K5. J Appl Bacteriol 1995;79:79-86.
- Mongomery HAC, Dymock JF. The determination of nitrite in water. Analyst 1962;86:414-416.
- Lowry OH, Rosebrough NJ, Farr AL, et al. Protein measurement with the folin-phenol reagent. J Biol Chem 1951;193:265-275.
- Fermanian C, Lapeyre C, Fremy J, et al. Diarrhoeal toxin production at low temperatures by selected strains of Bacillus cereus. J Dairy Res 1997;64:551-559.
- Finlay WJJ, Logan NA, Sutherland AD. Bacillus cereus produces most emetic toxin at lower temperatures. Letters in Applied Microbiology 2000;31:385-389.
- Shukor MY, Gusmanizar N, Azmi NA, et al. Isolation and characterization of an acrylamide-degrading Bacillus cereus. J Environ Biol 2009;30:57-64.
- Mills AL, Alexander M. N-Nitrosamine formation by cultures of several microorganisms. Appl Environ Microbiol 1976;31:892-895.
- Kunisaki N, Matsuuru H, Hayashi M. Formation of N-Nitrosodimethylamine by Escherichia coli. Food Hygiene and Safety Science (Shokuhin Eiseigaku Zasshi) 1976;17:314-319.
- Fournier D, Hawari J, Halasz A, et al. Aerobic Biodegradation of N-Nitrosodimethylamine by the Propanotroph rhodococcus ruber ENV425. Appl Environ Microbiol 2009;75:5088-5093.
- Ohhata N, Yoshida N, Egami H, et al. An extremely oligotrophic bacterium, Rhodococcus erythropolis N9T-4 isolated from crude oil. J Bacteriol 2007;189:6824-6831.
- An integrated risk information system for N-nitrosodimethylamine; CASRN 62-75-9. United State Environmental Protection Agency, USEPA, 2006.
- Garber E, Hollocher T. 15N, 18O tracer studies on the activation of nitrite by denitrifying bacteria. Journal of Biological Chemistry 1982;257:8091-8097.
- Bedzyk L, Wang T, Ye RW. The periplasmic nitrate reductase in Pseudomonas species strain G-179 catalyzes the first step of Denitrification. J Bacteriol 1999;181:2802-2806.
- Miler JH. Experiments in molecular genetics. P354. Cold spring Harbor, Laboratory Press, New York , 1972.
- Cleland WW. The kinetics of enzyme-catalyzed reactions with two or more substrates or products I. Nomenclature and rate reactions. Biochim Biophys Acta 1963;67:104-137.
- Cleland WW. The kinetics of enzyme-catalyzed reactions with two or more substrates or products II. Inhibition, nomenclature and theory. Biochim Biophys Acta 1963;67:173-187.
- Palmer T. Enzymes: Biochemistry, Biotechnology and Clinical Chemistry. Affliated East-West Press Pvt Limited, New Delhi, 2004.