Pulmonary Arteriovenous Malformation with Severe Mitral Valve Stenosis
Received: 30-Jun-2022, Manuscript No. PULCLR-22-5120; Editor assigned: 01-Jul-2022, Pre QC No. PULCLR-22-5120(QC); Reviewed: 15-Jul-2022 QC No. PULCLR-22-5120; Revised: 29-Aug-2022, Manuscript No. PULCLR-22-5120(R); Published: 05-Sep-2022, DOI: 10.37532/pulclr.2023.4(1).1-2
Citation: Agarwal SV. Pulmonary arteriovenous malformation with severe mitral valve stenosis. J Chest Lung Res 2022;3(5):1-2.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Pulmonary Arteriovenous Malformations (PAVM) is abnormal vascular connections between the pulmonary arterial and pulmonary venous circulations having a direct capillary free communication. This creates a Right to Left (R-L) shunt. PAVMs can be either congenital or acquired. A 38 year old female known case of rheumatic heart disease presented with complaint of dyspnea on exertion for 3 days. Her past medical record showed history of balloon mitral valvuloplasty twice for mitral stenosis and hypothyroidism.
Keywords
Pulmonary arteriovenous; Actinomycosis; Hepatic cirrhosis; Embolotherapy, Hypothyroidism
Introduction
Pulmonary Arteriovenous Malformations (PAVM) is abnormal vascular connections between the pulmonary arterial and pulmonary venous circulations having a direct capillary free communication. This creates a Right to Left (R-L) shunt. PAVMs can be either congenital or acquired. More than 80% of PAVMs are congenital among which the majority 47%-80% are associated with Osler Weber Render disease or hereditary haemorrhagic telangiectasia. Secondary or acquired PAVM, although very rare, has been reported in the literature. The causes of secondary PAVM include chest trauma, thoracic surgery, long standing hepatic cirrhosis, metastatic carcinoma, mitral stenosis, infections (actinomycosis, schistosomiasis), and systemic amyloidosis.
Case Presentation
A 38 years old female known case of rheumatic heart disease presented with complaint of dyspnea on exertion for 3 days. Her past medical record showed history of balloon mitral valvuloplasty twice for mitral stenosis and hypothyroidism. At presentation patient was a febrile and hypoxic with SpO2 77% at room air and respiratory rate of 22 breaths/min. Oxygen supplementation was given through oxygen mask still no improvement was seen in oxygen saturation. Physical examination showed bilateral upper and lower limb clubbing and cyanosis along with cyanosis of tongue and lips. Suspecting right to left shunting of blood, 2 D Echocardiography (ECHO) was done which showed thickened mitral valve and severe mitral stenosis (valve area 0.98 cm2 ). The left atrium was dilated and a thrombus (20 × 10 mm size) was seen attached to the septum. Moderate pulmonary artery hypertension and mild tricuspid regurgitation was also present. Contrast ECHO revealed a delay of 8 cycles when the bubbles was seen in left atrium hence suggesting a PAVM. Hence Contrast Enhanced Computed Tomography (CECT) chest was advised which revealed bilateral multiple PAVM. Patient was operated and mitral valve replacement was done with 29 mm SJMV. Patient refused for further surgical treatment of PAVM in view of high risk.
Results and Discussion
PAVM has an incidence of 2-3 per 100,000 population [1]. Sloan and Cooley in their 15,000 consecutive autopsies found only three cases of PAVM [2]. Incidence of 4.3 cases per year, 194 cases of PAVM was reported by Mayo Clinic over 45 years [3]. The male to female ratio varies from 1:1.5 to 1.8, in several series. Majority of cases are diagnosed in the first three decades of life.
The estimates of frequency with which PAVMs are due to HHT have varied substantially, from 36% to 95%. The acquired causes being mitral stenosis, amyloidosis, long standing hepatic cirrhosis, metastatic lesions, chest trauma and previous cardiac surgeries (glen operation) [4].
PAVMs may be single or multiple in occurrence and the incidence of single PAVMs ranges from 42% to 74%. Usually the involvement of solitary PAVMs are seen in bilateral lower lobes, the left lower lobe being the most common location, followed by right lower lobe, left upper lobe, right middle lobe, and right upper lobe. The majority of multiple PAVMs are also confined to bilateral lower lobes; the incidence of bilateral PAVMs ranges from 8% to 20% [5].
Symptoms related to PAVMs typically begin during the fourth through sixth decades of life, whereas symptoms of HHT frequently develop before the age of 20 years eg, epistaxis due to nasal telangiectasia or appearance of telangiectasia on the skin and lips [6]. Pulmonary symptoms include dyspnea on exertion, with a frequency ranging from 27% to 71%. Other symptoms due to PAVM include the following: hemoptysis (10%), chest pain (6%), finger clubbing (20%), cyanosis (18%) and thoracic murmurs (3%). Platypnea and orthodeoxia are also commonly encountered. Transient Ischemic Attack (TIA) occurs in up to 57% of patients with PAVM, and symptomatic cerebrovascular accident in 18% [7].
Radiologically PAVMs appear as oval, round, or bilobular pulmonary nodules. Contrast echocardiography is usually done to evaluate the shunt. It involves injection of agitated saline or dye into a peripheral vein and is extremely sensitive in detecting left to right shunt. It does not provide quantitative or anatomic detail of the shunt. In case of PAVM there is a delay of three to eight cardiac cycles before the bubbles are visualized in the left atrium [8]. The vascular anatomy of PAVM can be studied using contrast enhanced computed tomography [9]. Remy, et al. demonstrated that computed tomography scanning was significantly better than conventional angiography in detecting a PAVM (98% vs. 60%). Pulmonary angiography, although it is the most invasive tool for detection, remains the gold standard for confirming diagnosis of PAVM. It is usually required for patients who are candidates for resection or embolotherapy.
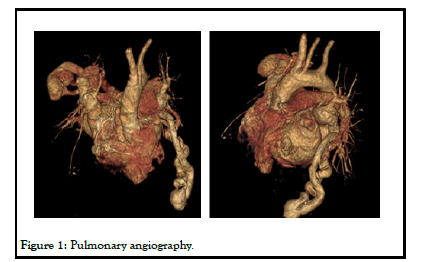
The morbidity was high (50%) when associated with PAVM among untreated patients compared with 3% in patients who received treatment. PAVMs with feeding vessel diameter of 3 mm or larger are embolized [10-12]. Embolization therapy has become the procedure of choice with success rates greater than 93%, and with very few serious complications and minimal loss of pulmonary parenchyma. There is no exposure to anaesthesia or thoracotomy (Figure 1) [13].
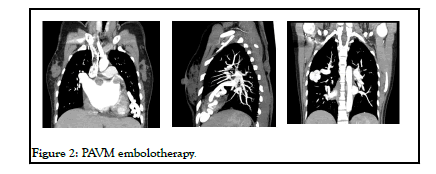
Till date only 14 cases of PAVM combined with rheumatic heart disease have been reported, among which only 4 were treated simultaneously by surgery. In our patient mitral valve replacement was done and was planned for PAVM embolotherapy on follow up (Figure 2).
Conclusion
Patient presenting with RHD coexisting with systemic arterial desaturation should be thoroughly evaluated. The classic triad of dyspnea on exertion, cyanosis and clubbing should alert the physician to the possibility of PAVM.
Hence after considering location of PAVM, size of the feeding artery and the draining vein choice of operation should be taken.
References
- Gossage James R, Ghassan K. Pulmonary arteriovenous malformation, A state of art review. Am J Respir Crit Care Med. 1998;158(2):643–61.
[crossreff] [Googlescholar] [Indexed]
- Sloan RD, Cooley RN. Congenital pulmonary arteriovenous aneurysm. Am J Roentgenol Radium Ther Nucl Med. 1953;70(2):183–210.
[Googlescholar] [Indexed]
- Swanson KL, Prakash UB, Stanson AW, et al. Pulmonary arteriovenous fistulas: mayo clinic experience, 1982-1997. Mayo Clin Proc. 1999; 74(7):671-80.
[crossreff] [Googlescholar] [Indexed]
- Khurshid I, Downie GH. Pulmonary arteriovenous malformation. Postgrad Med. 2002;78(918):191-7.
- Saboo SS, Chamarthy M, Bhalla S, et al. Pulmonary arteriovenous malformations: diagnosis. Cardiovasc Diagn Ther. 2018;8(3):325-37.
- Vase P, Holm M, Arendrup H. Pulmonary arteriovenous fistulas in hereditary hemorrhagic telangiectasia. Acta Med Scand. 1985;218(1):105–9.
[crossreff] [Googlescholar] [Indexed]
- Cottin V, Chinet T, Lavole A, et al. Groupe d'Etudes et de Recherche sur les Maladies 'Orphelines' Pulmonaires (GERM'O'P). Pulmonary arteriovenous malformations in hereditary hemorrhagic telangiectasia: a series of 126 patients. Medicine (Baltimore). 2007;86:1–17.
- Prager RL, Law KH, Bender HW J, et al. Arteriovenous fistula of the lung. Ann Thorac Surg. 1983;26:231–9.
- Hodgson CH, Kaye RL. Pulmonary arteriovenous fistula and hereditary telangiectasia. Dis Chest. 1963;43:449–55.
[crossreff] [Googlescholar] [Indexed]
- Dines DE, Arms RA, Bernatz PE, et al. Pulmonary arteriovenous fistulas. Mayo Clin Proc. 1974;49:460–5.
- Sluiter-Eringa H, Orie NGM, Slutier HJ. Pulmonary arteriovenous fistula: diagnosis and prognosis in non-complaint patients. Am Rev Respir Dis. 1969;100(2):177–84.
[crossreff] [Googlescholar] [Indexed]
- Pierce JA, Reagan WP, Kimball RW, et al. Unusual cases of pulmonary arteriovenous fistulas, with a note on thyroid carcinoma as a cause. N Engl J Med. 1959;260(18):901–7.
[Googlescholar] [Indexed]
- Kondoh H, Sato F, Shintani H. Simultaneous treatment of pulmonary AV malformation with rheumatic heart disease. Thorac Cardiovasc Surg. 2009; 57(3):174-6.
[Googlescholar] [Indexed]