The content of antioxidants - Phenolic compounds, in red wines of Georgia “Kindzmarauli” and “Saperavi”
Received: 17-May-2018 Accepted Date: May 28, 2018; Published: 31-May-2018
Citation: Kekelidze N, Kekelidze T, Akhalbedashvili L, et al. The content of antioxidants - Phenolic compounds, in red wines of Georgia “Kindzmarauli†and “Saperaviâ€Â. Appl Food Sci J. 2018;2(2):18-22.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
The aim of the study was to determine the qualitative and quantitative composition of phenolic compounds (PC) in different parts of grape Sapheravi (stem, skin, pulp, and seed) and in wines, obtained by Kakhetian and European methods using high-performance liquid chromatography (HPLC). The red wines Kindzmarauli and Saperavi contain the following PC: chlorogenic acid, caffeic, vanillic, lilac, wax acids, lilac aldehyde, quercetin- 3-β- D-glucoside, vanillin, catechins, myricetin, quercetin, resveratrol, kempferol. Gallic, ferulic and p-coumaric acids were not found. The total content of PC in skin extracts and wine were lower than in seed and stem more than in ten times, more part of PC in stems and seeds are phenolic acids, and a less quantity of flavonoids. The content of resveratrol in studied wines changes from 7.9 to 18.6 mg/L, and is equal 200.3 mg/L for stem of Saperavi grape, 338 mg/L in skin grape and 273 in seeds. A smaller number of catechins in wine, obtained by Kakhetian method in comparison with European one, indicate on more intensive oxidation process in Qvevri.
Keywords
Red wine; Phenolic compounds; Fermentation method; Qvevri; HPLC
The mechanism of action of the most common antioxidants (aromatic amines, phenols, naphthols, etc.) is that the reaction chains break: the antioxidant molecules interact with the active radicals to form low-activity radicals. Oxidation is also slowed down in the presence of substances that destroy hydroperoxides (dialkyl sulfides, etc.). In this case, the rate of formation of free radicals decreases. Even in a small amount (0.01-0.001%), antioxidants reduce the rate of oxidation, therefore, during a certain period of time (the period of inhibition, induction), oxidation products are not detected. In the practice of inhibition of oxidative processes, the phenomenon of synergy is of great importance - mutual enhancement of the effectiveness of antioxidants in the mixture or in the presence of other substances [1].
In biological systems, antioxidants are substances that can inhibit processes of free radical oxidation. For living cells, the most dangerous is the chain oxidation of polyunsaturated fatty acids, or lipid peroxidation (LPO). In LPO reactions, a large number of lipid hydroperoxides are formed, which have a high reactivity and have a powerful damaging effect on the cell.
Protection of the body against these and many other diseases is the main task of the antioxidant system [1-3]. They prevent lipid peroxidation and do not allow free radicals to accumulate in the body. However, the natural antioxidant system of the body is often overloaded and literally choked with an avalanche of free radicals. This condition is called oxidative stress. Most often, oxidative stress is caused by UV radiation, which not only induces free radical oxidation, but also disrupts the work of enzyme antioxidants of the skin.
Deficiency of endogenous antioxidants can be replenished by natural biologically active compounds, especially phenolic compounds, contained in beverages and food products. These compounds have the physiological function in plants: promote plant adaptation to environment, improve their resistance to pesticides and diseases, and influence on characteristics of fruits, in particular color, taste, fragrance, and transparency. Indirect influence of phenolic compounds on aroma and bouquet of wines is manifested in the fact that due to their participation in oxidative deamination of amino acids various aldehydes with a pleasant odor are formed.
Taste and other quality indicators of wine depend not only on the total content of phenolic substances, but also on their physicochemical state. In most cases, young wines have a high content of unoxidized forms of tannin, which gives them a strong astringent taste. As a result of oxidation of tannins with aging of wines, their taste becomes softer with velvet shade.
Such an important qualitative indicator of wines as color is determined by the content of mono- and polymeric phenolic compounds passing from grape during its processing. The intensity of coloring the young wine and the formation of its different shades depends on the quality of the anthocyanins, their physicochemical composition, pH of the medium, the content of SO2 and other factors.
The special attention of researchers is attracted by grape wines - sources of a unique natural composition of antioxidants and other biologically active substances, superior in their activity to individual antioxidants. Most of the components of the phenolic complex of grapes and wine belong to the group of biologically active substances, thanks to which red wines are increasingly used to treat numerous diseases. Grapes, especially its red and black kinds, except taste qualities, the content of a large number of easily assimilable mono- and disaccharides, is the richest source of phenolic compounds, and also 3- and 4- hydroxystilbenes. Polyphenols are found not only in the pulp of grapes, but also in the skin of fruits, in seeds, in branches of grape bunch, and during the preparation of wine they do not collapse. About 50 components of the phenolic complex (especially resveratrol, kvercetine, rutine) exhibit radioprotective, anti-radiation, bactericidal, antioxidant, antisclerotic, neurostimulating, tonic and other functional properties. They are divided into two main subgroups: flavonoids (catechins, proanthocyanidins, flavonols and anthocyanins) and non-flavonoids (hydroxybenzoic acids, hydroxycinnamic acids and stilbens) [4-6].
Increased interest in red wines, especially canteens, compared with white, is not accidental. They contain more the natural antioxidants, ensuring the prevention of mentioned diseases. According to European legislation, the content of phenolic compounds in white wine should be at least 150 mg/L, in red - at least 600 mg/L. Among a large number of identified phenolic compounds, a special place belongs to resveratrol, the chemical name of which is 3,5,4’- trihydroxy-trans-stylbene. It is a natural phytoalexin, which is formed in grapes under extreme conditions [7,8]. Resveratrol is presented in grapes and wines in trans- and cis- forms. Trans-resveratrol is more stable, contained in higher concentrations and is mainly responsible for biochemical and antibiotic effects. According to Baraboy [9] the highest concentration of resveratrol (5.13 mg/mL) is in the wine Pino noir. Along with resveratrol in the tissues of grapes another phytoalexin is synthesized, a close analogue - piceannol, differing the presence of the fourth hydroxyl group in the molecule. It has the majority of biological effects of trans-resveratrol [10].
The amount of resveratrol in grapes depends on the type of grapes (presence of infection, soil, climate, environmental pollution, processing technology, storage. In grapes resveratrol is found mainly in the skin, a small amount (5-7 times less) is in the seeds and even less (10-50 times) - in the pulp [11]. All this determines the high importance of red wines in the human diet.
Chemical analyzes of Georgian wines also showed a high content of phenolic compounds in them, especially in red wines obtained by the Kakhetian traditional method - aging in “Qvevri”.
This circumstance prompted us to investigate and compare the content of some flavonoids, phenolic acids, flavonols and resveratrol in the different parts of Saperavi grapes and red wines of Kindzamarauli and Saperavi from the eastern regions of Georgia, manufactured by traditional Kakhetian and European methods. The vintage wines of Kindzamarauli and Saperavi and the corresponding raw materials from Sabue and Mukuzani vineyards were kindly provided by the wine enterprise Khareba. Earlier we determined the mineral composition and content of heavy metals in these grape and wines [12,13].
Materials and Methods
Methanol, ethanol, acetonitrile, acetic acid, ethyl acetate, chemical HPLCgrade standards of trans-resveratrol, gallic acid, caffeic acid, p-coumaric acid, (+)-catechin, (-)-epicatechin, vanillic acid, ferulic acid, kaempferol, quercetin, rutin, and myricetin were obtained from Sigma with 97-99% purity used were in analytical reagent grade and purchased from Sigma Aldrich Company Ltd., Great Britain.
Samples of Saperavi grapes were gathered for analysis in Mukuzani and Sabue vineyards in Kakheti (Eastern Georgia) in autumn instantly after vintage at the stage of technical maturity. The stems, leaves, skins, and seeds were scoured by hand, washed under running water and dried at the room temperature.
The analyzes were carried out using the high-performance liquid chromatography method on an Agilent Technologies 1260 chromatograph equipped with a gradient pump, automatic device to reject the sample, an ultraviolet detector, and a computer data management and processing system. Detection was made at 280 nm. As the chromatographic column for division of components, Agilent-Eclipse XDB-C18 with 15 μm grain size was used. As eluents, acetonitrile with 99.9% purity, acetic acid, bidistillate were used. The flow rate of the mobile phase was 1 mL/min. As mobile phases were used the mixture of acetic acid and bidistilled water in ratio 2:98, and acetonitrile/ bidistilled water/acetic acid in ratio 90:8:2. The volume of the sample was 20 μL. A 60% solution of ethanol in water was used for the extraction.
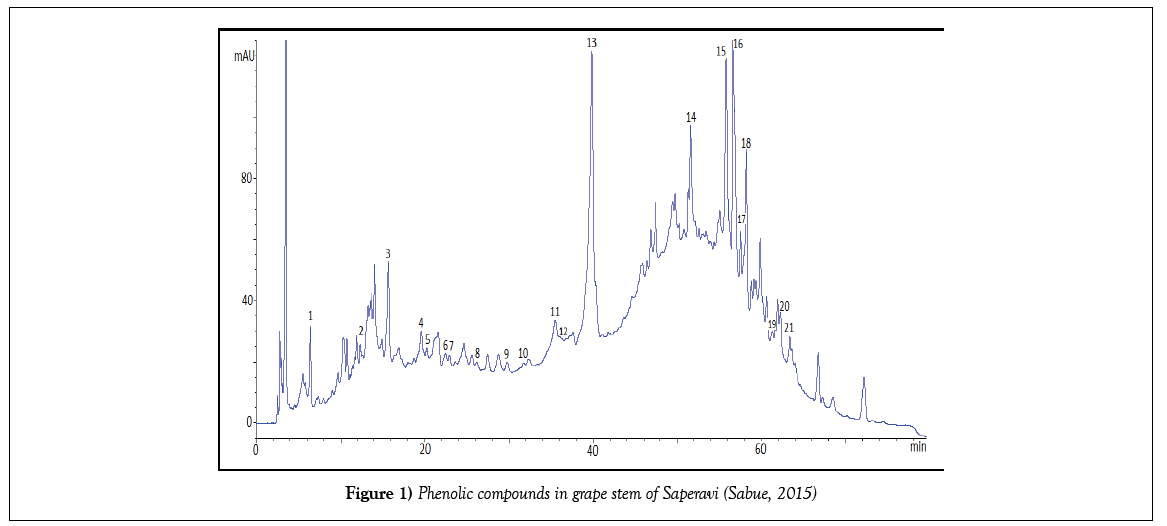
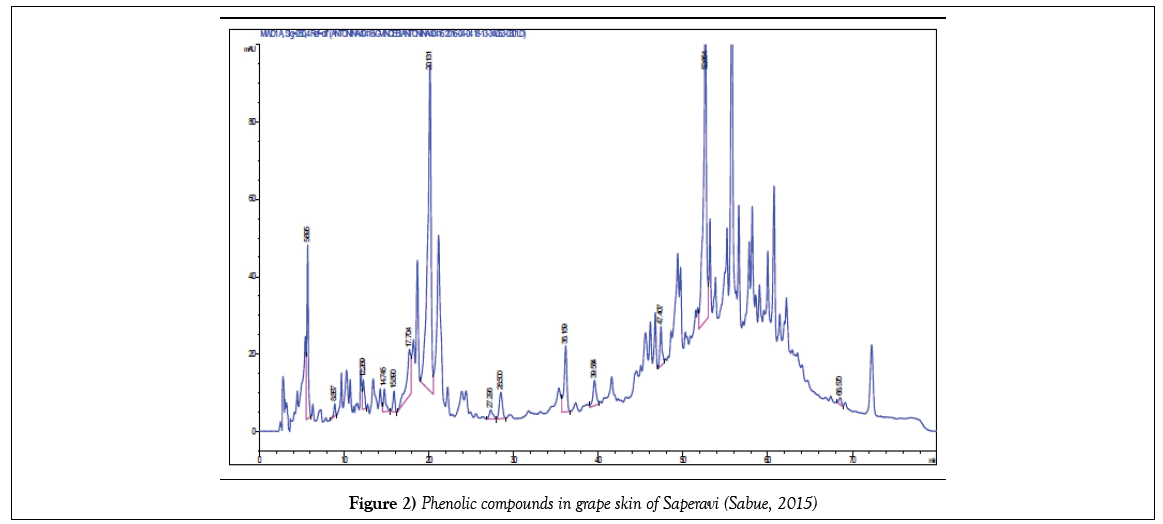
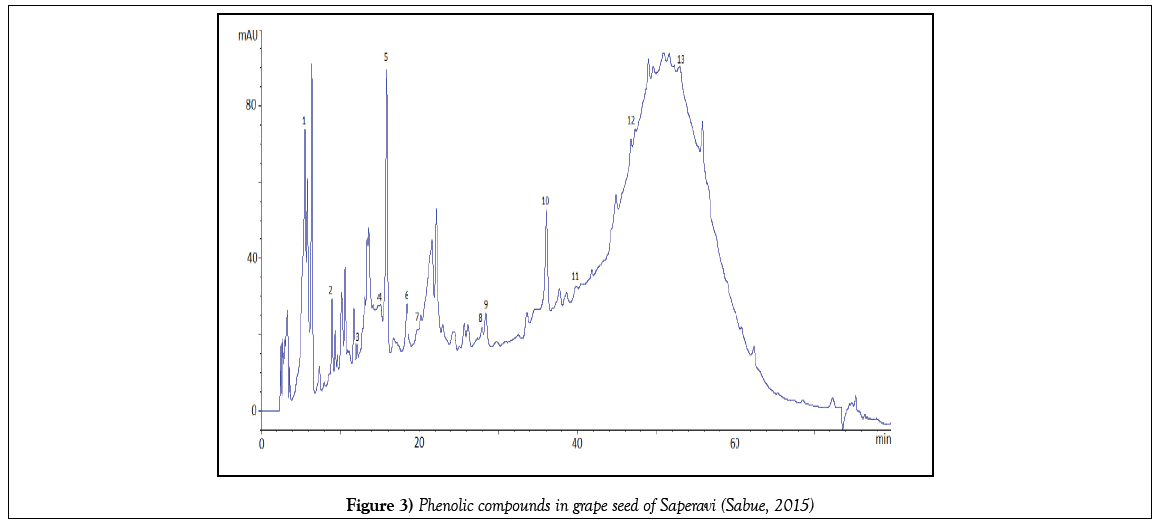
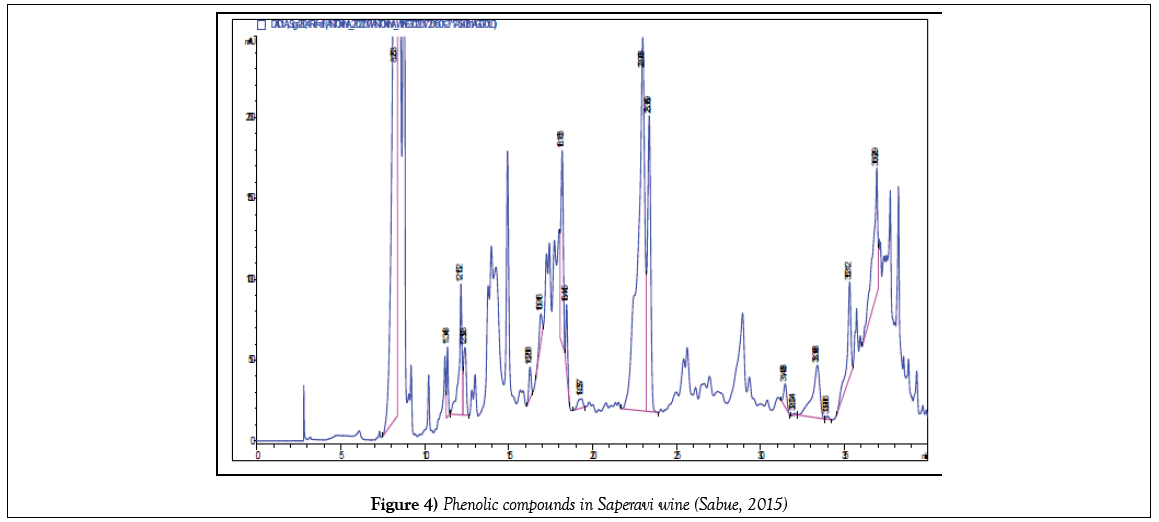
The exact quantity of grape sample (stem, skin, pulp, seeds) weighing 1 g during 24 hours was kept in 10 mL of a 60% solution of ethanol. After a fivefold extraction, the solution filtered and quantitatively transferred to a 50 mL volumetric flask. The alcohol was then distilled under vacuum, thereby leaving phenolic compounds in the aqueous phase. From the aqueous phase, they were extracted with ethyl acetate, the resulting phase was dried with sodium sulfate, the ethyl acetate was removed under vacuum and the resulting residue was dissolved in methanol. Prior to the analysis, they were filtered on a 0.45 μm membrane filter. Chromatographic separation was carried out as follows: gradient elution with bidistillate and acetonitrile, aciditated with acetic acid. Total analysis time is no more than 60 minutes. The phenolic compounds were identified using retention times of appropriate standards. For quantification calculation the calibration curves were constructed for the identified compounds. All measurements were made thrice. Chromatograms of some identified phenolic compounds are submitted below (Figures 1-4).
Results and Discussion
As it is known, wine is possessed the primary and secondary aromas [14]. The primary aroma depends on the grape sort, and the secondary one is formed in the process of alcohol fermentation under the yeast influence.
The bouquet of aromas is created during the wine maturation as a result of the complex biochemical transformation of both aromas. These specific aromas are depended on carbohydrates splitting with formation of aldehydes, lactones, volatile phenols [15] also at low concentration. Exactly phenolic substances affect the color, taste and bouquet of wines, on their resistance to the development of certain microorganisms harmful to wines. They are among the most easily oxidized constituents of grapes and wine and are very labile. The hard parts of grapes, especially stems and seeds play the significant role in formation of aromas and taste of wine due to their rich chemical composition.
Therefore, we found it necessary to trace the change in the content of phenolic substances in the hard parts of the vine and compare it with their content in wines.
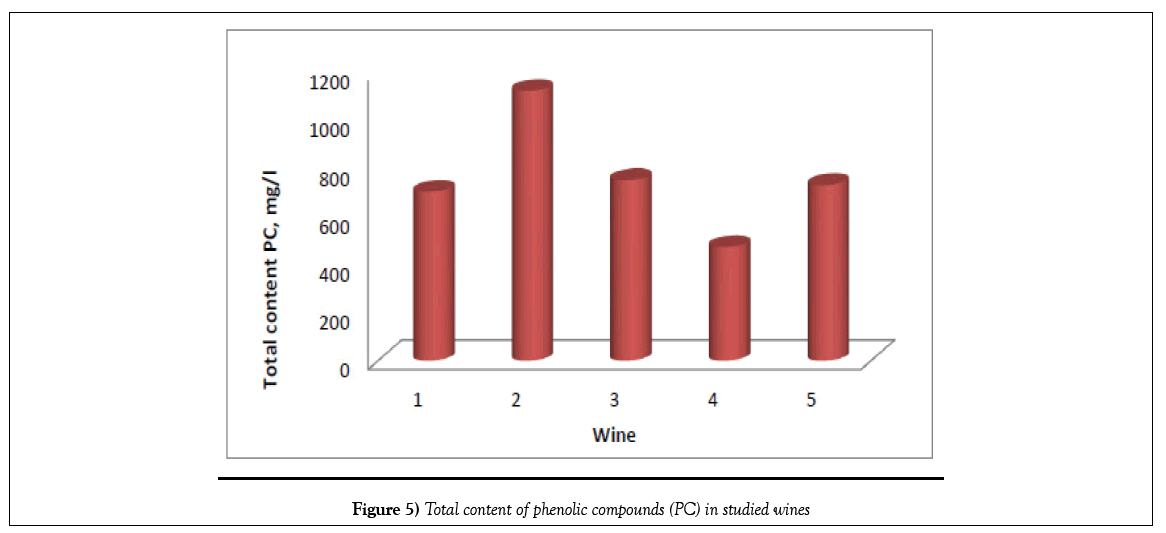
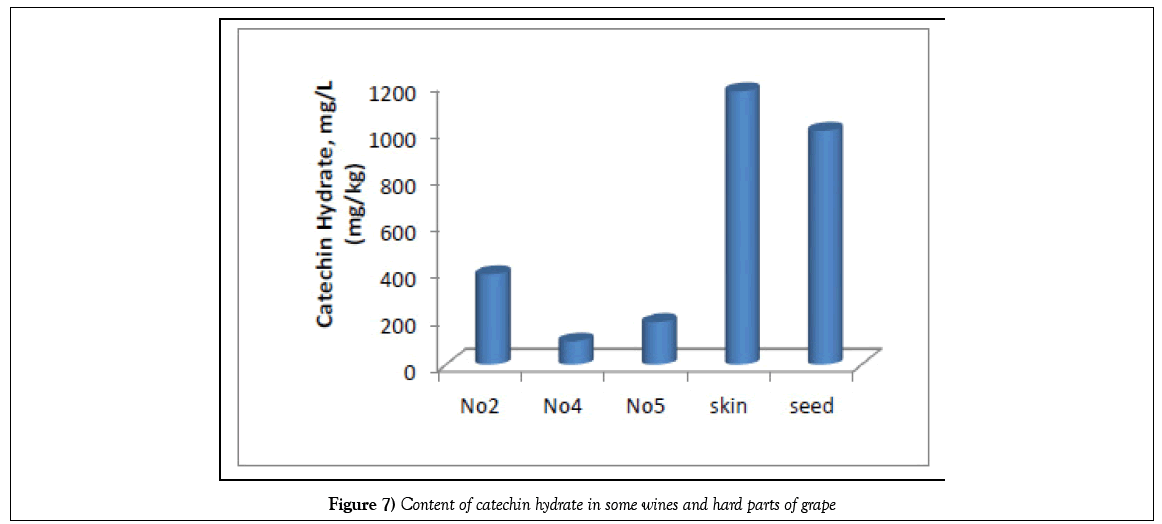
The experimental data (Tables 1 and 2) (Figure 5) show that the total content of phenolic compounds is highest in Saperavi wine, kept in Qvevri over the wort - 1121 mg/L (sample No. 2), compared to the wines of Kindzmarauli and Saperavi, obtained from grape juice by industrial method (703 and 752 mg/L for samples No. 1 and 3, respectively). If compare Saperavi samples according to years, the lowest content of phenolic compounds is observed for wine obtained from the 2012 crop - 475 mg/L. The content of catechins in vintage wines of enterprise Khareba is varied in limits 220 - 420 mg/L, and the content of catechins is more-420 mg/L than in other studied wines, the content of identified phenolic acids in wine No 2 is also more and is equal about 600 mg/L. So, Qvevri wine, obtained by maturation of crushed grapes in clay jug, is richer with phenolic acids and catechins, than wines, obtained by fermentation of grape wort in barrels or cisterns.
| No. | Wine | Method of making | Region of origin | Year of making |
|---|---|---|---|---|
| 1 | Kindzmarauli | Industrial method | Kakheti, Mukuzani | 2015 |
| 2 | Saperavi | Qvevri method | Kakheti, Sabue | 2015 |
| 3 | Saperavi | Industrial method | Kakheti, Sabue | 2010 |
| 4 | Saperavi | Industrial method | Kakheti, Sabue | 2012 |
| 5 | Saperavi | Industrial method | Kakheti, Sabue | 2014 |
Table 1: List of studied wine
| No. | Component (mg/L) | No.1 | No.2 | No.3 | No.4 | No.5 |
|---|---|---|---|---|---|---|
| 1 | Gallic acid | nd | nd | nd | nd | nd |
| 2 | Catechin Hydrate | 221.084 | 387.729 | 272.086 | 100.526 | 181.320 |
| 3 | Wax acid | 124.710 | 335.825 | 84.307 | 35.659 | 223.685 |
| 4 | Chlorogenic acid | 78.220 | 137.179 | 69.763 | 35.566 | 109.902 |
| 5 | Ferulic acid | nd | nd | nd | nd | nd |
| 6 | p-coumaric acid | nd | nd | nd | nd | nd |
| 7 | Epicatehin | 118.454 | 31.495 | 25.160 | 20.150 | 37.816 |
| 8 | Caffeic acid | 55.608 | 16.852 | 7.158 | 6.892 | 80.552 |
| 9 | Vanillic acid | 5.861 | 8.316 | 1.886 | 4.556 | 6.653 |
| 10 | Vanillin | 10.504 | 9.1541 | 45.321 | 49.015 | 17.390 |
| 11 | Lilac acid | 3.566 | 66.030 | 8.117 | 7.181 | 2.895 |
| 12 | Lilac aldehyde | 63.188 | 51.149 | 121.388 | 131.283 | 15.284 |
| 13 | Quercetin-3-β-D-glucoside | 7.075 | 4.265 | 14.321 | 12.793 | 9.837 |
| 14 | Myricetin | 63.469 | 0 | 69.352 | 0 | 0 |
| 15 | Luteolin | 1.115 | 0.752 | 0.562 | 0.751 | 0.955 |
| 16 | Quercetin dihydrate | 14.747 | 55.413 | 23.268 | 52.639 | 35.488 |
| 17 | Apigenin | 0 | 0 | 0.774 | 1.061 | 0 |
| 18 | Kempferol | 0.818 | 3.175 | 1.374 | 0.423 | 2.472 |
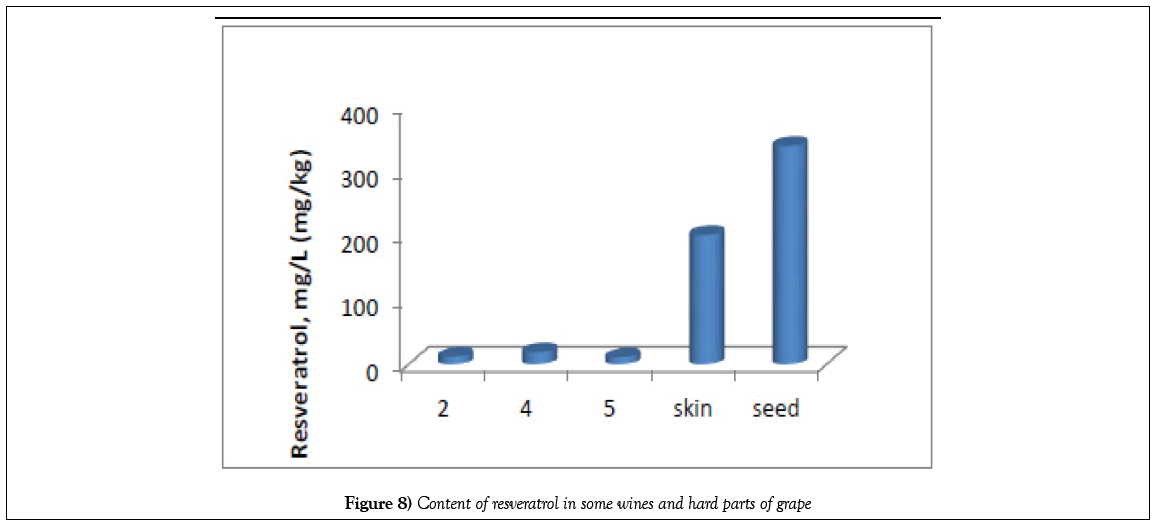
| 19 | Resveratrol | 7.921 | 11.629 | 10.140 | 18.656 | 10.475 |
| 20 | Total content | 703 | 1121 | 752 | 475 | 731 |
Table 2: Content of identified phenolic compounds in red wines Kindzmarauli and Saperavi
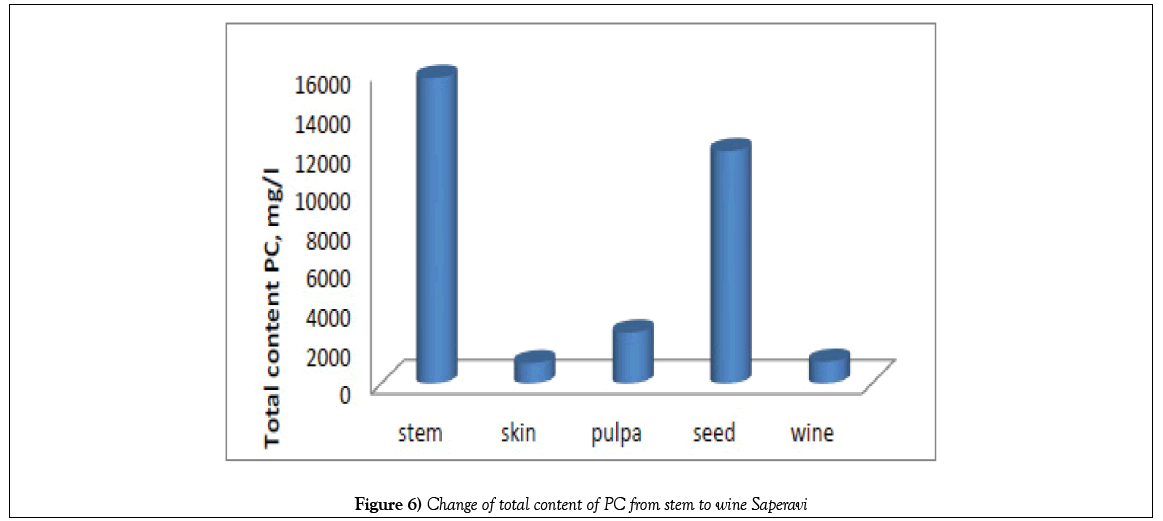
But the total content of PC in skin extracts and wine were lower than in seeds and stems more than in ten times (Figure 6), at the same time more part of PC in stems and seeds are phenolic acids, and a little quantity of flavonoids. As it was expected, PC is found mainly in the hard parts of the grape bunch – seeds and stems.
In common the following hydroxybenzoic acids were found in hard parts of grape and wines Kindzmarauli and Saperavi: сhlorogenic acid, сaffeic, vanillic, lilac acids, and in large quantities lilac aldehyde and wax acid. As opposed to Brazil, Argentine and Chile wines (varieties Pinot Noir, Cabernet Sauvignon, Merlot and others) [16] studied wine don’t contain gallic, ferulic, p-coumaric acids, but they contain such compounds as chlorogenic acid, lilac acid, lilac aldehyde, quercetin-3-β-D-glucoside, and vanillin, which are absent in South American wines.
The content of vanillic acid in South American wines varied in limits 0.00- 1.154 mg/L, in Georgian wines 1.89-8.32 mg/L, caffeic acid varied in limits 2.74-4.95 mg/L in South American wines and from 6.89 to 80.55 mg/L in Georgian red wines, and the content of trans-resveratrol changes in limits 1.56-4.30 mg/L to compare with 7.92-18.65 mg/L for Kindzmarauli and Saperavi. The content of catechin hydrate, wax acid, lilac and chlorogenic acids are maximum in wine №2, obtained according the Qvevri method. Perhaps, it is the peculiarity of studied grape Saperavi and wines. It is obvious that the phenolic compound contents varied not only within grape varieties and used technology, but between countries too. According to McDonald et al. [17] the content of phenols in red wines in different countries sometimes reaches 4.0 - 4.5 g/L.
According to the diagrams obtained (Figures 6-8), the total content of phenolic acids, as well as catechins and resveratrol, sharply decreases from the skin and grape seeds to ready-made wine. The content of these substances in solid parts is almost an order of magnitude greater than their quantity in pulp, juice, and even more so wine. At the same time, the content of catechin in wine, obtained by the Qvevri method is more than twice as high as that in wine, obtained by the European method.
A tenfold increase the content of phenolic acids in the solid parts of the grapes compared to pulp, juice and wine indicates that the phenolic acids, during maturation and further fermentation, are converted into flavonoids, whose content in wine is higher than in the hard parts of the grapes. But most of acids removed from wine in the form of a sediment [18].
The natural antioxidant - resveratrol, a compound with multiple health benefits, was found in all wine samples and its amount were in some times more or comparable with the concentration range found in the literature [19- 21]. The authors Zakharova A, Kravchenko A and Ivanova A [22] affirmed that exception is red dry wine from blending of grape varieties Cabernet Sauvignon and Merlot (produced in France)-24.7 mg/L, but in samples from other regions of the globe, its number sometimes less than an order of magnitude. For example, the lowest content of trans-resveratrol was found in red dry wine from the blend of Cabernet Sauvignon and Merlot grape varieties (produced in Chile) - 0.8 mg/L.
The amount of resveratrol in grapes depends on the type of grapes (presence of infection (fungal), soil, climate, environmental pollution, processing technology, storage [23,24].
In grapes, resveratrol is found mainly in the skin, a small amount (5-7 times less) is in the seeds and else smaller (10-50 times) - in the pulp [25], but transresveratrol is contained in the red grapes juice in the range 0.01-1.1 ppm, and cis-resveratrol is within 0.003-0.23 ppm [26]. This fact partially agrees with our data - the content of resveratrol in the studied Saperavi variety and the corresponding wines in individual cases is 1000 mg/L (Mukuzani wineyard) for the skin, 200 for the stem, 28 for pulp and 11-18 mg/L for wines. Thus, the wines studied are distinguished by a rather high content of resveratrol, which confirms the naturalness of these wines. It should be noted that the presence of trans-resveratrol in drinks confirms the natural origin of wines.
Last year’s winemakers considered content of resveratrol as indicator of red wine’s quality. In freshly prepared and very old wines this parameter is small. Because in studied wines this parameter is more than 7 mg/L, Kindzmarauli and Saperavi wines of winery “Khareba” are of class “very good”, but “Saperavi” of 2012 group is refers to class “outstanding”.
Conclusion
Based on the results of the analysis of wines and initial wine materials and comparing them with some literature data, we concluded that the geographic location, climate, soil type, and especially the grape kind, and processing technology, have a determining effect on the content of phenolic compounds in the Saperavi wines studied. So, the peculiarity of these wines is the absence of gallic, ferulic, p-coumaric acids in contrast to other red wines, a significant content of flavonols and a lower content of phenolic acids in wines than in skin and seeds.
Thus, phenolic acids are actively involved in all stages of fermentation of wine. The content of one of the most important components - resveratrol, is consistently high for all the wines studied, at the same time the amount of resveratrol is inversely proportional to the content of phenolic acids. A smaller number of catechins in wine, obtained by the Kakhetian method in comparison with the European one, indicate on more intensive oxidation process in Kvevri. A biological activity of Kakhetian wines is determined by the P-vitamin activity of (-) epicatechins.
Acknowledgements
This work was supported by Shota Rustaveli, National Science Foundation (SRNSF), [DI/38/7-220/14, the complex study of antioxidants and mineral components in Georgian red wines by modern physical-chemical methods.
REFERENCES
- Guendez R, Kallithraka S, Makris DP, et al. Determination of low molecular weight polyphenolic constituents in grape (Vitis vinifera sp.) seed extracts: correlation with antiradical activity. Food Chem. 2005;89(1):1-9.
- Monagas M, Bartolome B, Gomez-Cordoves C. Updated knowledge about the presence of phenolic compounds in wine. Crit Rev Food Sci Nutr. 2005;45(2):85-118.
- Shahidi F, Wanasundara PK. Phenolic antioxidants. Crit Rev Food Sci Nutr. 1992;32(1):67-103.
- Ali K, Maltese F, Choi YH, et al. Metabolic constituents of grapevine and grape-derived products. Phytochem Rev. 2010;9(3):357-78.
- Iriti M, Faoro F. In: Bioactive foods in promoting health. Academic Press. 2010;58(1):620.
- Fernandez-Marin MI, Guero FR, Puertas B, et al. In: Natural Products: Phytochemistry, Botany and metabolism of Alkaloids, Phenolics and Terpenes. Springer-Verlag. Berlin. 2013:25810-2615.
- Wang Y, Cotana F, Yang Y, et al. An LC-MS Method for Analyzing Total Resveratrol in Grape Juice, Cranberry Juice, and in Wine. J Agr Food Chem. 2002;50(3):431-35.
- Gilly R, Mara D, Oded S, et al. Resveratrol and a novel tyrosinase in carignan grape juice. J Agr Food Chem. 2001;49(3):1479-85.
- Baraboy VA. Phenolic compounds of the vine: Structure, antioxidant activity, application. Biotechnology. 2009;2(2):67-75.
- Wolter F, Claushitzer A, Akaglu B, et al. Piceatannol, a natural analog of resveratrol, inhibits progression through the S phase of the cell cycle in colorectal cancer cell lines. J Nutr. 2002; 132(2):298-302.
- Jeandet P, Bessis R, Gautheron B. The production of resveratrol (3,5,4’-trihydroxystilbene) by grape berries in different developmental stages. Amer J Enology and Viticulture. 1991;42(1):41-6.
- Kekelidze N, Kekelidze T, Akhalbedashvili L, et al. Some mineral components in Georgian red wines. Science of Europe. 2017;12(12):4-8.
- Kekelidze N, Kekelidze T, Akhalbedashvili L, et al. Heavy metals in Georgian red wines Saperavi and Kindzmarauli. 13th International Congress on Advances in Natural Medicines Nutraceuticals and Neurocognition. Rome, Italy. 2017:43.
- Riberi-Gaion Zh, Payn E, Riberi-Gaion N, et al. The Theory and practice of winemaking. M., “Pishchepromizdat”. 1979:48.
- Pisarnitski AF. Aroma-forming substances of wines, cognacs. Abstract of Doctorate Dissertation (in Rus.) 1980.
- Granato D, Chizuko F, Katayama U, et al. Phenolic composition of South American red wines classified according to their antioxidant activity, retail price and sensory quality. Food Chem. 2011;129(2):366-73.
- McDonald MS, Hughes M, Burns J, et al. Survey of the free and conjugated myricetin and quercetin content of red wines of different geographical origins. J Agric Food Chem. 1998;46(2):368-75.
- Glonti T, Glonti Z. The remarkable Qvevri wine. Tbilisi, 2013:58
- Badamtestseg S, Hoza I, Valбšek P, et al. Determination of phenolic compounds in Moravian wines. Mongolioan J Chem. 2012;13(39):37-40.
- Rastija V, Srečnik G, Šarić MM, Polyphenolic composition of Croatin wines with different geographical origins. Food Chem. 2009;115(1):54-60.
- Gerogiannaki-Christopoulou M, Athanasopoulos P, Kyriakidis N, et al. Trans-resveratrol in wines from the major Greek red and white grape varieties. Food Control. 2006;17:700-6.
- Zakharova A, Kravchenko A, Ivanova A, et al. The comparative analysis of dry red wine by HPLC and atom-emission spectroscopy methods. Analytica. 2017;2(33):86-96.
- Wang Y, Cotana F, Yang Y, et al. An LC-MS method for analyzing total resveratrol in grape juice, cranberry juice and in wine. J Agr Food Chem. 2002;50(3):431-5.
- Gilly R, Mara D, Oded S, et al. Resveratrol and a novel tyrosinase in carignan grape juice. J Agr Food Chem. 2001;49(3):1479-1485.
- Jeandet P, Bessis R, Gautheron B. The production of resveratrol (3,5,4'-trihydroxystilbene) by grape berries in different developmental stages. Am J Enol Vitic. 1991;42:41-6.
- Romero-Perez AI. Piceid, the major resveratrol derivative in grape juices. J Agr Food Chem. 1999;47(4):1533-36.