
Page 29
J Can Res Metastasis, Volume 3
September 16-17, 2019 | Edinburgh, Scotland
Volume 3
Breast Cancer 2019 & Cancer Science 2019
September 16-17, 2019
Journal of Cancer & Metastasis Research
BREAST CANCER
CANCER SCIENCE AND THERAPY
2
nd
World Congress on
&
Combination therapies with epigenetic inhibitors for the treatment of soft tissue
sarcomas
Zoë Walters
University of Southampton, UK
D
ifferentiation therapy is an approach that has had notable success in a limited number of cancer types including acute
promyelocytic leukaemia and neuroblastoma but remains underexplored for most tumour types. Therapeutic drugs
have been shown to promote differentiation in preclinical models including soft tissue sarcomas (STS) that often resemble
undifferentiated mesenchymal tissues. Rhabdomyosarcomas (RMS) are the most common paediatric STS and appear as
developing skeletal muscle that are unable to terminally differentiate through aberrant recapitulation of developmental programs.
Histone modifications are known to govern these developmental programs by controlling activities such as DNA transcription
and cell differentiation. Enhancer of Zeste Homolog 2 (EZH2) confers histone methyltransferase activity to the Polycomb
Repressive Complex 2 (PRC2), and is known to control stem cell renewal and differentiation.
We have shown that EZH2 and other members of the Polycomb Repressive Complex 2 (PRC2) play a role in the differentiation
program of RMS and are required to maintain the undifferentiated phenotype of these tumours. Single agent modulation of EZH2
using a tool compound or clinical drug candidate results in modest differentiation of RMS cell lines, a phenotype that we show
is augmented by combination with differentiating agents in vitro. Furthermore, we show that this combination can also be used
to effectively reduce proliferation in synovial sarcoma lines in vitro. Thus combining inhibition of histone modifying enzymes
with differentiating agents or other frontline therapies already in clinical use represent a novel potential avenue for therapeutic
intervention for use in the treatment of STS.
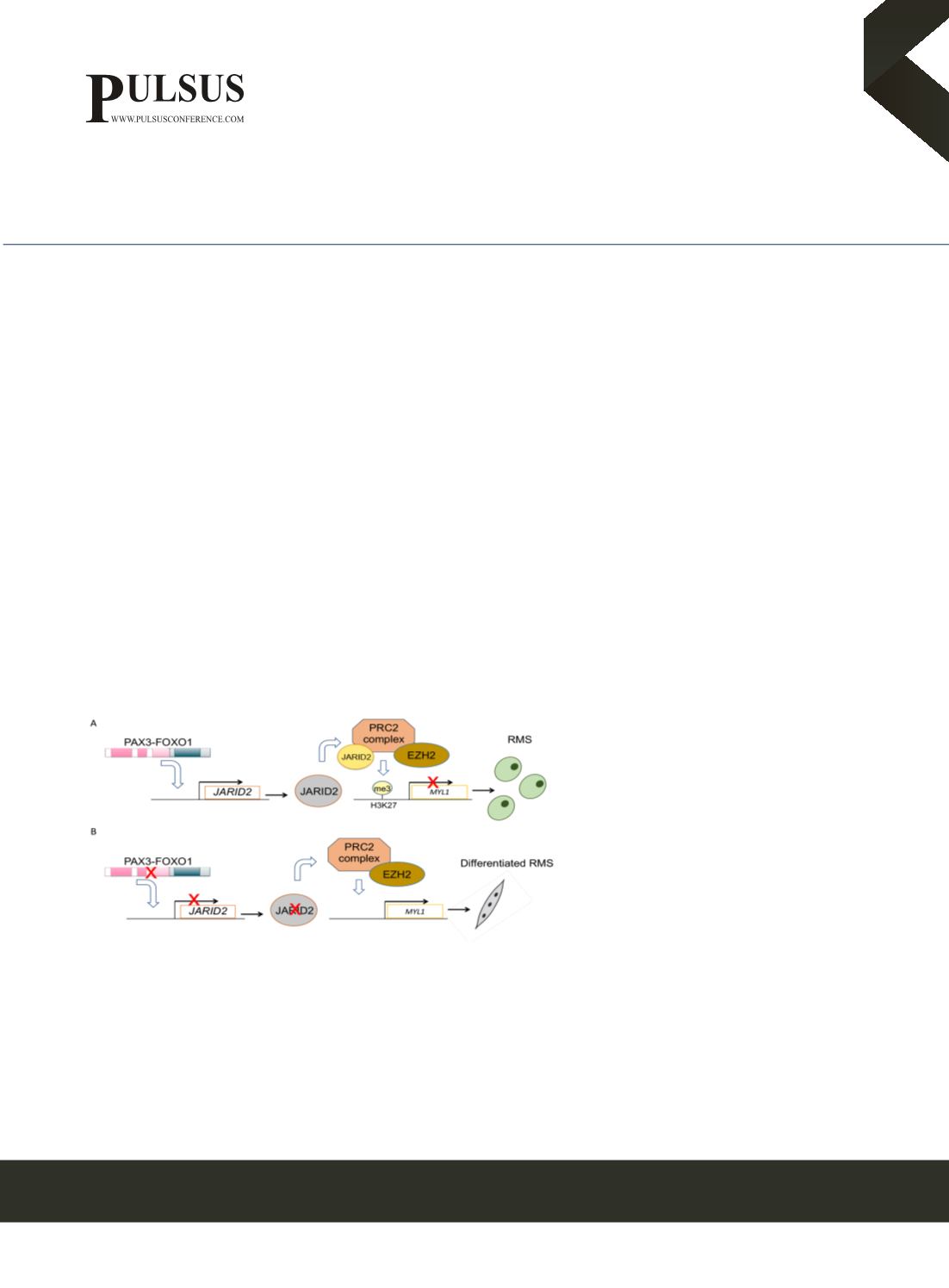
Z.S.Walters@soton.ac.ukThe Polycomb Repressive Complex 2 (PRC2) complex
maintains the undifferentiated state of rhabdomyosarcoma
(RMS) cells. (A) We have shown that the PAX3-FOXO1
fusion protein present in high-risk Alveolar RMS tumours
directly regulates the expression of JARID2, a member of
the PRC2 complex, which in turn methylates H3K27 on the
promoter of myogenic genes to maintain the proliferative,
undifferentiated state of RMS cells. (B) Silencing of
JARID2, and other members of the PRC2 complex,
results in removal of these methyl marks and ultimately to
differentiation of RMS cells to a more benign state.
















