
Page 26
Volume 2
July 24-25, 2019 | Rome, Italy
World Hematology 2019 & Nursing Care 2019
July 24-25, 2019
Journal of Blood Disorders and Treatment
47
th
WORLD CONGRESS ON NURSING CARE
11
th
WORLD HEMATOLOGY AND ONCOLOGY CONGRESS
&
Two novel DNMT3Amutations in acute myeloid leukemia
Bruno S
1
, Bochicchio MT
2
, Franchini E
1
, Padella A
1
, Pazzaglia M
1
, Raffini M, Bandini L, Venturi C
1
, Simonetti G
2
,
Soverini S
1
, Ottaviani M
1
and Martinelli G
2
1
University of Bologna, Italy
2
Istituto Scientifico Romagnolo Per Lo Studio e la Cura dei Tumori, Italy
Statement of the Problem
: Recurrent somatic mutations of DNMT3A
occur in about 20% of acute myeloid leukemia (AML) patients,
targeting a hot spot site at R882 codon [1]. DNMT3A mutations in
primary AML samples are often heterozygous and are associated with
CpG hypomethylation [2], which result in high myeloblast counts, and
poor prognosis [3]. The study aims to characterize two new mutations
in the DNMT3A gene, identified in two AML patients.
Methodology & Theoretical Orientation
: DNA was extracted from
mononucleated cells and it was sequenced by Sanger Sequencing
and Next-Generation sequencing. Sequences obtained were mapped
to human reference genome GRCh37/hg19 and annotated using Ion
Reporter 5.10.2.0. The Methyl Flash Methylated DNA Quantification
Kit was used to detect CpG methylation status. DNMT3A protein level was assessed by western blot.
Findings
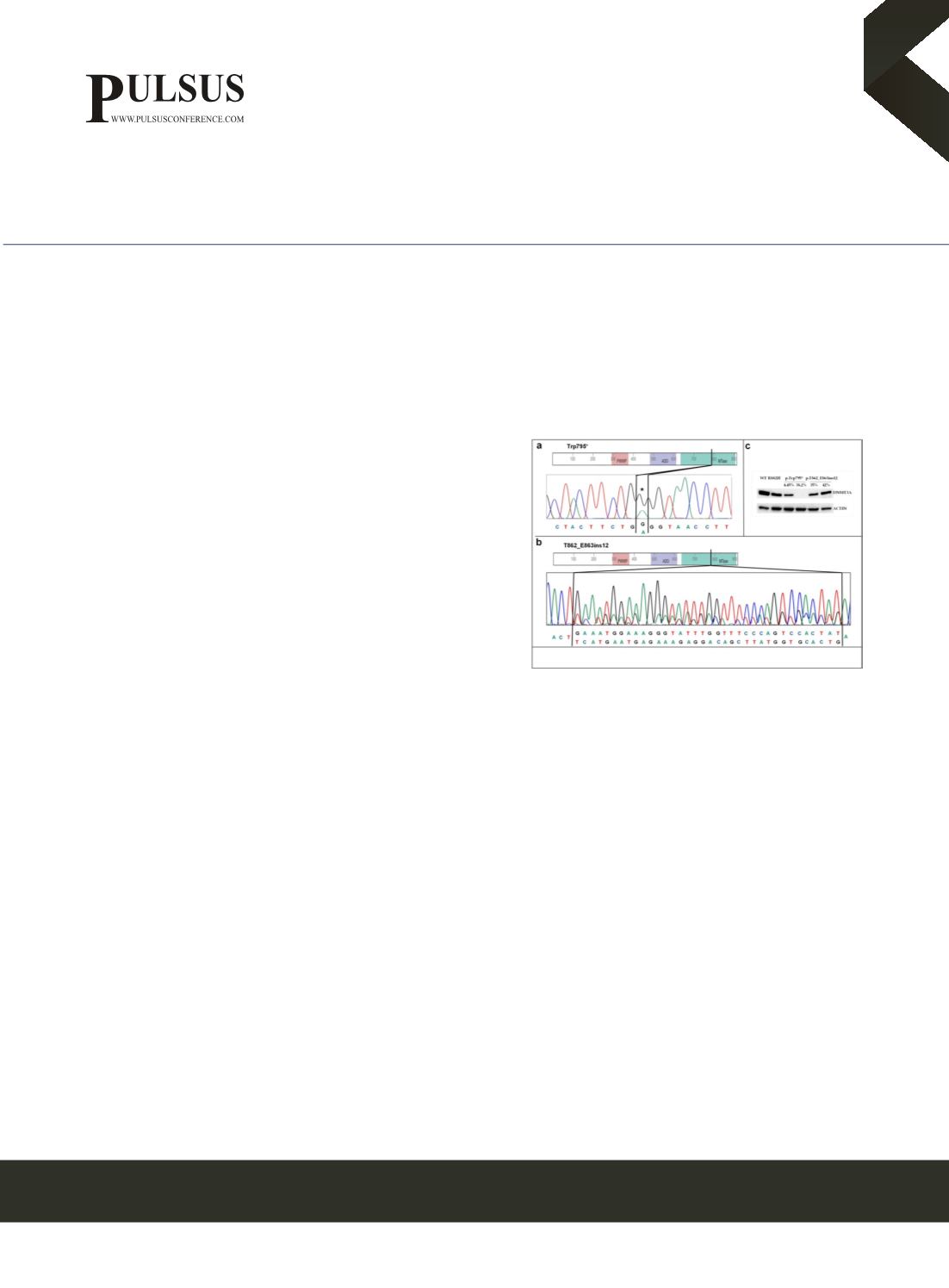
: Patient #1 had 70% of blasts in the BM at diagnosis and showed an undescribed single nucleotide variant of
DNMT3A at exon 20 causing a premature STOP codon (cDNA c.2385G>A; tgG/tgA p. Trp795*; NM_022552;), coupled
with IDH2 R172K mutation. The DNMT3A mutation load increased from 4% in the diagnosis sample to 38.2% in the follow-
up, which had stable disease, evaluated 4 months after treatment in multicentric clinical trial. The increase of mutation rate
correlated with DNA hypo-methylation and lead to loss of protein expression. Patient #2 had 80% of blasts in the BM at
diagnosis and 90% at relapse, with a new insertion of 36 nucleotides in exon 22 of the DNMT3A gene (c.2924_2925ins36:
TCATGAATGAGAAAGAGGACATCTTATGGTGCACT), along with FLT3-ITD. DNMT3A mutation load was 27.5% at
diagnosis and increased at 48% at relapse, which occurred 7 months after completion of chemotherapy treatment. We did not
observe a significant variation of protein levels, neither of DNA methylation.
Conclusion & Significance
: Obtained data support the hypothesis that DNMT3A mutations may be involved in pre-leukemic
clonal hematopoietic expansion.
Biography
Samantha Bruno is a PhD student in hematology in university of Bologna. She has her expertise in molecular and cellular biology.
Her research is mainly focused on molecular characterization of acute myeloid leukemia primary sample and in vitro study in order to
identify new drugs for personalized therapy of acute myeloid leukemia patients.
samantha.bruno2@unibo.itJ Blood Disord Treat, Volume 2
















